-
PDF
- Split View
-
Views
-
Cite
Cite
Montserrat VilÀ, Alison M Dunn, Franz Essl, Elena GÓmez-DÍaz, Philip E Hulme, Jonathan M Jeschke, MartÍn A NÚÑez, Richard S Ostfeld, AnÍbal Pauchard, Anthony Ricciardi, Belinda Gallardo, Viewing Emerging Human Infectious Epidemics through the Lens of Invasion Biology, BioScience, Volume 71, Issue 7, July 2021, Pages 722–740, https://doi.org/10.1093/biosci/biab047
Close - Share Icon Share
Abstract
Invasion biology examines species originated elsewhere and moved with the help of humans, and those species’ impacts on biodiversity, ecosystem services, and human well-being. In a globalized world, the emergence and spread of many human infectious pathogens are quintessential biological invasion events. Some macroscopic invasive species themselves contribute to the emergence and transmission of human infectious agents. We review conceptual parallels and differences between human epidemics and biological invasions by animals and plants. Fundamental concepts in invasion biology regarding the interplay of propagule pressure, species traits, biotic interactions, eco-evolutionary experience, and ecosystem disturbances can help to explain transitions between stages of epidemic spread. As a result, many forecasting and management tools used to address epidemics could be applied to biological invasions and vice versa. Therefore, we advocate for increasing cross-fertilization between the two disciplines to improve prediction, prevention, treatment, and mitigation of invasive species and infectious disease outbreaks, including pandemics.
Invasive species (i.e., non-native, alien, exotic species) that have been introduced to new regions by humans, that form self-sustaining populations, and that spread rapidly from the sites of introduction; (Blackburn et al. 2011, Essl et al. 2018) can have enormous impacts on the environment, the economy, and human well-being (Vilà and Hulme 2017, Pyšek et al. 2020). Invasion biology, a discipline examining the ecological, evolutionary, and anthropogenic processes involved in the spread and impact of non-native species, has mostly been focused on free-living, conspicuous macroscopic species, whose spread is observable and easy to track. In contrast, the invasion dynamics of parasites and pathogens have received less attention, except for those causing damage to agriculture, forestry, and livestock (but see Mallon et al. 2015, Thakur et al. 2019, Pyšek et al. 2020). More recently, the focus has expanded to include pathogens that affect wildlife (Hatcher et al. 2012, Dunn and Hatcher 2015, Roy et al. 2017). Human infectious agents that rapidly increase in incidence and geographic area can also be viewed as a biological invasion but have rarely been treated as such (Hatcher et al. 2012, Nuñez et al. 2020), although many studies have described the direct and indirect human health impacts of biological invasions, including those involving the introduction of human pathogens (Hatcher et al. 2012, Rabitsch et al. 2017).
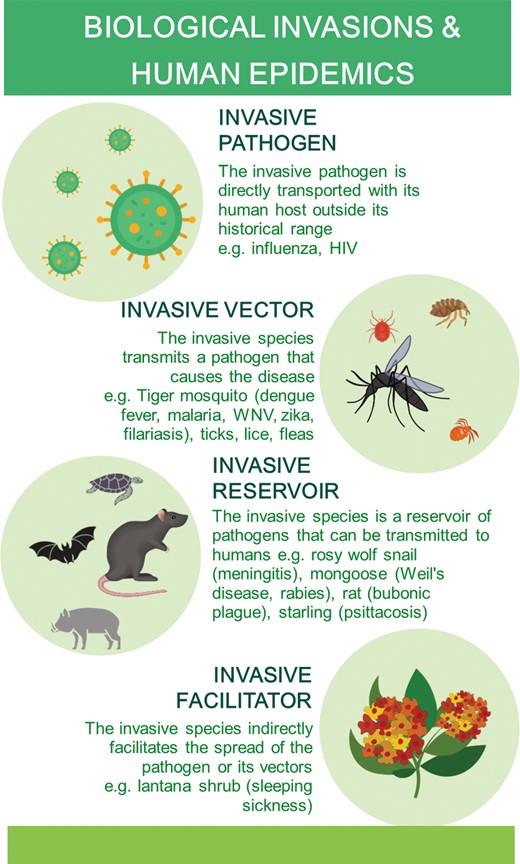
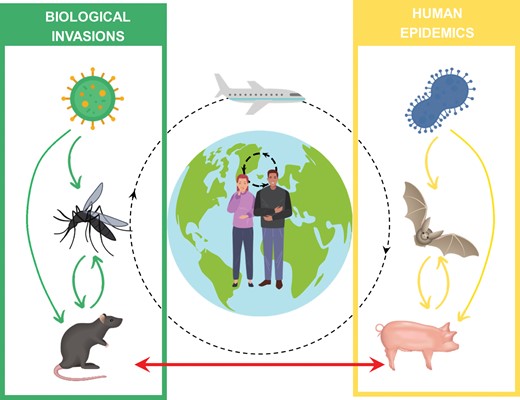
A human pathogen can spread beyond its historical range and become invasive, usually as a result of the movement of infected human hosts. In addition to humans assisting the spread of invasive animal and plant species, invasive species themselves can facilitate the large-scale propagation of human pathogens and epidemics by acting as vectors or reservoir hosts of emerging human pathogens or by providing habitat for them (figure 1). Indeed, 16% of the species on the IUCN list of 100 of the World's Worst Invasive Alien Species (Lowe et al. 2000) promote the spread and impact of human pathogens (table 1). Invasive insects are the most frequent vectors of pathogens causing human diseases (Lounibos 2002). For example, the tiger mosquito (Aedes albopictus) has spread to all inhabited continents through trade and is a vector of several infectious pathogens, including those causing dengue fever, yellow fever, West Nile virus, and chikungunya (Gratz 2004, Enserink 2008). Another group of invasive mosquitoes are some Anopheles species, the most important vectors of Plasmodium species, the blood parasites that cause malaria (Lounibos 2002, Takken and Lindsay 2019). Invasive vertebrates such as rodents are frequent reservoirs or intermediate hosts of human pathogens (Hatcher et al. 2012, Hulme 2014a). Finally, invasive species, particularly plants, can create habitat conditions conducive to local proliferation of vector or reservoir hosts (Mack and Smith 2011, Rai and Singh 2020). For example, the invasive bush Lantana camara attracts and provides refuge for tsetse flies away from river courses and close to villages, promoting sleeping sickness epidemics (Syed and Guerin 2004). Similarly, water hyacinth (Eichhornia crassipes) forms dense mats that provide breeding habitat for mosquitoes that transmit Plasmodium (causative agent of malaria), Filofilaria immitis (filariasis), or Flavivirus species (dengue fever; Mack and Smith 2011). These cases exemplify the enormous diversity of combinations of native–invasive pathogen, host, and reservoir that are possible (figure 2), suggesting myriad potential roles of invasive species in the ecology and global spread of pathogens (Rabitsch et al. 2017).
Human emerging diseases can be caused directly by invasive pathogens, by pathogens transported by invasive vectors or reservoirs, or facilitated by invasive species not directly involved in the life cycle or transportation of the pathogen, but rather promoting the presence and abundance of its vectors and reservoirs. For examples, see table 1.
Interplay between biological invasions and human emerging infectious diseases. Pathogen transmission can be within invasive species (left), within native or livestock species (right) and across invasive and native species (the bottom arrow). Dashed arrows indicate pathogen transmission to humans within a population (the small circle) or globally (the large circle).
Species from the IUCN 100 of the World's Worst Invasive Alien Species List that can transmit pathogens to humans or are themselves pathogens.
| Invasive species . | Pathogens (diseases) . | Transmission . | Pathways . | Impacts . |
|---|---|---|---|---|
| Acridotheres tristis, common myna | Ornithonyssus bursa and Dermanyssus gallinae (dermatitis, skin inflammation, severe irritation and rashes, asthma) | Reservoir | Intentional or escape from confinement: zoo, pet trade | A, B, H |
| Their droppings can spread psittacosis, ornithosis, salmonellosis and arboviruses. | Intentional or release in nature: fauna “improvement” | |||
| Aedes albopictus, tiger mosquito | Flavivirus spp. (e.g., West Nile, dengue fever, yellow fever), Dilofilaria immitis (filariasis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Achatina fulica, giant African land snail | Metastrongylus spp., Angiostrongulus cantonensis and Angiostrongulus costaricensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or escape from confinement: pet, aquarium and terrarium species, research, horticulture, live food | H, A |
| Anopheles quadrimaculatus, mosquito | Plasmodium spp. (malaria), West Nile virus (meningoencephalitis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Eichhornia crassipes, water hyacinth | Plasmodium spp. (malaria) transmitted by annopheline mosquitoes | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: aquarium species | A, B, H |
| Eriocheir sinensis, Chinese mitten crab | Paragonimus westermanii (human lung fluke parasite), | Reservoir | Intentional or escape from confinement: aquaculture, aquarium species. | A, B, H |
| Unintentional or transport stowaway: ship or boat ballast water, ship or boat hull fouling | ||||
| Euglandina rosea, rosy wolf snail | Angiostrongylus cantonensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or release in nature: biological control | B, H |
| Herpestes javanicus, small Indian mongoose | Leptospira interrogans (Weil's disease), Lyssavirus (rabies) | Reservoir | Intentional or release in nature: biological control | B, H |
| Lantana camara, lantana shrub | Tripanosoma spp. (sleeping sickness) transmitted by Glossina spp., tsetse fly | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: horticulture | A, B, H |
| Macaca fascicularis, crab-eating macaca | Macacine herpesvirus 1 (herpes B), Lyssavirus (rabies) | Reservoir | Intentional or escape from confinement: live food, research | A, B, H |
| Mus musculus, house mouse | Yersinia pestis (bubonic plague), Salmonella spp. (salmonelosis) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Rattus rattus, black rat | Leptospira interrogans (Weil's disease), Yersinia pestis (bubonic plague) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Sturnus vulgaris, starling | Chlamydophila psittaci (psittacosis) | Reservoir | Intentional or release in nature: biological control, hunting, fauna “improvement” | A, H |
| Sus scrofa, feral pig | Leptospira interrogans (Weil's disease) | Reservoir | Intentional or release in nature: hunting | A, B, H |
| Trachemys scripta elegans, red eared slider turtle | Salmonella spp. (salmonelosis) | Reservoir | Intentional or escape from confinement: aquarium and terrarium species | A, B, H |
| Vulpes vulpes, red fox | Possible role in Lyssavirus (rabies) transmission | Reservoir | Intentional or release in nature: hunting | A, B, H |
| Invasive species . | Pathogens (diseases) . | Transmission . | Pathways . | Impacts . |
|---|---|---|---|---|
| Acridotheres tristis, common myna | Ornithonyssus bursa and Dermanyssus gallinae (dermatitis, skin inflammation, severe irritation and rashes, asthma) | Reservoir | Intentional or escape from confinement: zoo, pet trade | A, B, H |
| Their droppings can spread psittacosis, ornithosis, salmonellosis and arboviruses. | Intentional or release in nature: fauna “improvement” | |||
| Aedes albopictus, tiger mosquito | Flavivirus spp. (e.g., West Nile, dengue fever, yellow fever), Dilofilaria immitis (filariasis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Achatina fulica, giant African land snail | Metastrongylus spp., Angiostrongulus cantonensis and Angiostrongulus costaricensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or escape from confinement: pet, aquarium and terrarium species, research, horticulture, live food | H, A |
| Anopheles quadrimaculatus, mosquito | Plasmodium spp. (malaria), West Nile virus (meningoencephalitis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Eichhornia crassipes, water hyacinth | Plasmodium spp. (malaria) transmitted by annopheline mosquitoes | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: aquarium species | A, B, H |
| Eriocheir sinensis, Chinese mitten crab | Paragonimus westermanii (human lung fluke parasite), | Reservoir | Intentional or escape from confinement: aquaculture, aquarium species. | A, B, H |
| Unintentional or transport stowaway: ship or boat ballast water, ship or boat hull fouling | ||||
| Euglandina rosea, rosy wolf snail | Angiostrongylus cantonensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or release in nature: biological control | B, H |
| Herpestes javanicus, small Indian mongoose | Leptospira interrogans (Weil's disease), Lyssavirus (rabies) | Reservoir | Intentional or release in nature: biological control | B, H |
| Lantana camara, lantana shrub | Tripanosoma spp. (sleeping sickness) transmitted by Glossina spp., tsetse fly | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: horticulture | A, B, H |
| Macaca fascicularis, crab-eating macaca | Macacine herpesvirus 1 (herpes B), Lyssavirus (rabies) | Reservoir | Intentional or escape from confinement: live food, research | A, B, H |
| Mus musculus, house mouse | Yersinia pestis (bubonic plague), Salmonella spp. (salmonelosis) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Rattus rattus, black rat | Leptospira interrogans (Weil's disease), Yersinia pestis (bubonic plague) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Sturnus vulgaris, starling | Chlamydophila psittaci (psittacosis) | Reservoir | Intentional or release in nature: biological control, hunting, fauna “improvement” | A, H |
| Sus scrofa, feral pig | Leptospira interrogans (Weil's disease) | Reservoir | Intentional or release in nature: hunting | A, B, H |
| Trachemys scripta elegans, red eared slider turtle | Salmonella spp. (salmonelosis) | Reservoir | Intentional or escape from confinement: aquarium and terrarium species | A, B, H |
| Vulpes vulpes, red fox | Possible role in Lyssavirus (rabies) transmission | Reservoir | Intentional or release in nature: hunting | A, B, H |
Note: The introduction pathways (according to the Convention of Biological Diversity) and impact types are indicated. Abbreviations: A, damage to human activities such as to agriculture, forestry, livestock or infrastructures; B, damage to biodiversity; H, damage to human health.
Species from the IUCN 100 of the World's Worst Invasive Alien Species List that can transmit pathogens to humans or are themselves pathogens.
| Invasive species . | Pathogens (diseases) . | Transmission . | Pathways . | Impacts . |
|---|---|---|---|---|
| Acridotheres tristis, common myna | Ornithonyssus bursa and Dermanyssus gallinae (dermatitis, skin inflammation, severe irritation and rashes, asthma) | Reservoir | Intentional or escape from confinement: zoo, pet trade | A, B, H |
| Their droppings can spread psittacosis, ornithosis, salmonellosis and arboviruses. | Intentional or release in nature: fauna “improvement” | |||
| Aedes albopictus, tiger mosquito | Flavivirus spp. (e.g., West Nile, dengue fever, yellow fever), Dilofilaria immitis (filariasis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Achatina fulica, giant African land snail | Metastrongylus spp., Angiostrongulus cantonensis and Angiostrongulus costaricensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or escape from confinement: pet, aquarium and terrarium species, research, horticulture, live food | H, A |
| Anopheles quadrimaculatus, mosquito | Plasmodium spp. (malaria), West Nile virus (meningoencephalitis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Eichhornia crassipes, water hyacinth | Plasmodium spp. (malaria) transmitted by annopheline mosquitoes | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: aquarium species | A, B, H |
| Eriocheir sinensis, Chinese mitten crab | Paragonimus westermanii (human lung fluke parasite), | Reservoir | Intentional or escape from confinement: aquaculture, aquarium species. | A, B, H |
| Unintentional or transport stowaway: ship or boat ballast water, ship or boat hull fouling | ||||
| Euglandina rosea, rosy wolf snail | Angiostrongylus cantonensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or release in nature: biological control | B, H |
| Herpestes javanicus, small Indian mongoose | Leptospira interrogans (Weil's disease), Lyssavirus (rabies) | Reservoir | Intentional or release in nature: biological control | B, H |
| Lantana camara, lantana shrub | Tripanosoma spp. (sleeping sickness) transmitted by Glossina spp., tsetse fly | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: horticulture | A, B, H |
| Macaca fascicularis, crab-eating macaca | Macacine herpesvirus 1 (herpes B), Lyssavirus (rabies) | Reservoir | Intentional or escape from confinement: live food, research | A, B, H |
| Mus musculus, house mouse | Yersinia pestis (bubonic plague), Salmonella spp. (salmonelosis) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Rattus rattus, black rat | Leptospira interrogans (Weil's disease), Yersinia pestis (bubonic plague) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Sturnus vulgaris, starling | Chlamydophila psittaci (psittacosis) | Reservoir | Intentional or release in nature: biological control, hunting, fauna “improvement” | A, H |
| Sus scrofa, feral pig | Leptospira interrogans (Weil's disease) | Reservoir | Intentional or release in nature: hunting | A, B, H |
| Trachemys scripta elegans, red eared slider turtle | Salmonella spp. (salmonelosis) | Reservoir | Intentional or escape from confinement: aquarium and terrarium species | A, B, H |
| Vulpes vulpes, red fox | Possible role in Lyssavirus (rabies) transmission | Reservoir | Intentional or release in nature: hunting | A, B, H |
| Invasive species . | Pathogens (diseases) . | Transmission . | Pathways . | Impacts . |
|---|---|---|---|---|
| Acridotheres tristis, common myna | Ornithonyssus bursa and Dermanyssus gallinae (dermatitis, skin inflammation, severe irritation and rashes, asthma) | Reservoir | Intentional or escape from confinement: zoo, pet trade | A, B, H |
| Their droppings can spread psittacosis, ornithosis, salmonellosis and arboviruses. | Intentional or release in nature: fauna “improvement” | |||
| Aedes albopictus, tiger mosquito | Flavivirus spp. (e.g., West Nile, dengue fever, yellow fever), Dilofilaria immitis (filariasis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Achatina fulica, giant African land snail | Metastrongylus spp., Angiostrongulus cantonensis and Angiostrongulus costaricensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or escape from confinement: pet, aquarium and terrarium species, research, horticulture, live food | H, A |
| Anopheles quadrimaculatus, mosquito | Plasmodium spp. (malaria), West Nile virus (meningoencephalitis) | Vector | Unintentional or transport stowaway: vehicles | H |
| Eichhornia crassipes, water hyacinth | Plasmodium spp. (malaria) transmitted by annopheline mosquitoes | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: aquarium species | A, B, H |
| Eriocheir sinensis, Chinese mitten crab | Paragonimus westermanii (human lung fluke parasite), | Reservoir | Intentional or escape from confinement: aquaculture, aquarium species. | A, B, H |
| Unintentional or transport stowaway: ship or boat ballast water, ship or boat hull fouling | ||||
| Euglandina rosea, rosy wolf snail | Angiostrongylus cantonensis (pulmonary metastrongylosis and eosinophilic meningoencephalitis) | Reservoir | Intentional or release in nature: biological control | B, H |
| Herpestes javanicus, small Indian mongoose | Leptospira interrogans (Weil's disease), Lyssavirus (rabies) | Reservoir | Intentional or release in nature: biological control | B, H |
| Lantana camara, lantana shrub | Tripanosoma spp. (sleeping sickness) transmitted by Glossina spp., tsetse fly | Invasive facilitator (habitat for vector) | Intentional or escape from confinement: horticulture | A, B, H |
| Macaca fascicularis, crab-eating macaca | Macacine herpesvirus 1 (herpes B), Lyssavirus (rabies) | Reservoir | Intentional or escape from confinement: live food, research | A, B, H |
| Mus musculus, house mouse | Yersinia pestis (bubonic plague), Salmonella spp. (salmonelosis) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Rattus rattus, black rat | Leptospira interrogans (Weil's disease), Yersinia pestis (bubonic plague) | Reservoir | Unintentional or transport stowaway: container, bulk | A, B, H |
| Sturnus vulgaris, starling | Chlamydophila psittaci (psittacosis) | Reservoir | Intentional or release in nature: biological control, hunting, fauna “improvement” | A, H |
| Sus scrofa, feral pig | Leptospira interrogans (Weil's disease) | Reservoir | Intentional or release in nature: hunting | A, B, H |
| Trachemys scripta elegans, red eared slider turtle | Salmonella spp. (salmonelosis) | Reservoir | Intentional or escape from confinement: aquarium and terrarium species | A, B, H |
| Vulpes vulpes, red fox | Possible role in Lyssavirus (rabies) transmission | Reservoir | Intentional or release in nature: hunting | A, B, H |
Note: The introduction pathways (according to the Convention of Biological Diversity) and impact types are indicated. Abbreviations: A, damage to human activities such as to agriculture, forestry, livestock or infrastructures; B, damage to biodiversity; H, damage to human health.
Both biological invasions and infectious diseases are becoming more prevalent and widespread with globalization. The two phenomena share common drivers of introduction and spread (Mack et al. 2000, Jeschke et al. 2013). In biological invasions, there has been a substantial amount of research on species traits conferring invasion potential (i.e., invasiveness), on the vulnerability of the ecosystems to be invaded (i.e., invasibility), and on the role of environmental conditions facilitating or preventing spread (Pyšek et al. 2012). Similarly, research on infectious diseases mainly focuses on understanding factors influencing the ability to establish persistent infections and cause disease (i.e., virulence) and on the transmission from host to host (i.e., transmission), why some microorganisms and specific strains cause disease, which individuals and human populations are more susceptible to infection, and how or which environmental conditions affect pathogen spread (Horrocks et al. 2011). However, because research on invasions and epidemics are approached by different disciplines, the bodies of literature and terminology are usually separated (box 1). An exchange and cross-fertilization between these two research domains is needed to advance the prevention, treatment, and adaptation of their impacts (Conn 2009, Ogden et al. 2019, Hulme et al. 2020, Nuñez et al. 2020).
Emerging infectious disease. An infectious disease that appears in a human population for the first time or has existed previously but is rapidly increasing in incidence, impact or geographic range (www.emro.who.int/health-topics/emerging-diseases/index.html).
Epidemic. A disease event affecting many people at the same time, and spreading from person to person in a locality or region during a specific period of time (www.who.int/csr/disease/swineflu/frequently_asked_questions/pandemic/en).
Invasive species. A non-native introduced species that form self-sustaining populations and spread rapidly from the sites of introduction (Blackburn et al. 2011).
Invasiveness. Intrinsic characteristics of a non-native species to invade outside its region of origin (Lonsdale 1999).
Invasibility. Susceptibility of an ecosystem to be invaded. It depends on the biotic and abiotic characteristics of the recipient ecosystem (Lonsdale 1999).
Non-native species. An introduced species transported intentionally or unintentionally to a new region by humans (Blackburn et al. 2011).
One Biosecurity. An interdisciplinary approach to biosecurity policy and research that builds on the interconnections between human, animal, plant, and environmental health to effectively prevent and mitigate the impacts of invasive alien species (Hulme 2021).
One Health. Cross-sectoral approach to achieve optimal public health outcomes by monitoring, managing and investigating the interactions between humans, animals, and their environments (Ogden et al. 2019).
Outbreak. The occurrence of more infection cases than expected in a particular population, in a specific geographical area and in a specified period (www.emro.who.int/health-topics/disease-outbreaks/index.html).
Pandemic. An epidemic occurring worldwide, or over a very wide area, crossing international boundaries and usually affecting a large number of people (www.who.int/csr/disease/swineflu/frequently_asked_questions/pandemic/en)
Pathogen pressure. Amount of pathogen available to the human host at a given point in space and time (Plowright et al. 2017).
Reservoir. An animal species that hosts a pathogen, typically without being harmed, and is the source of infection to other host species (Rabitsch et al. 2017).
Spillover. Transmission of a pathogen from a reservoir to a novel susceptible host (Rabitsch et al. 2017).
Time lag. Period between the introduction of a non-native species and its establishment in the new range. In the broad sense, it can be applied to the time required to overcome any phase of the invasion process (Crooks 2005).
Vector. A species, typically but not always an arthropod, that carries and transmits a pathogen to another species (Rabitsch et al. 2017).
Virulence. Ability of a microorganism to cause disease. It depends on characteristics of the pathogen and the host (Horrocks et al. 2011).
Zoonosis. A disease causing pathogen that is transmitted between vertebrate animals (wildlife, livestock or domestic animals) and humans (Rabitsch et al. 2017).
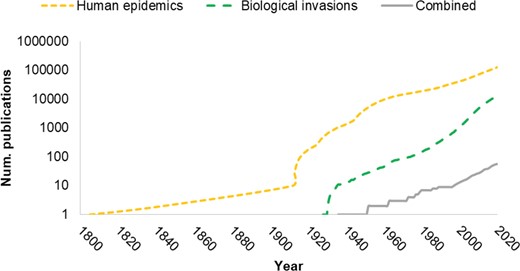
The introductions of invasive species and human pathogens have been described as co-occurring phenomena caused by the transport of species, including people, during early European colonization of the Americas and some African and Asian territories from the fifteenth to the seventeenth century (Crosby 2004, Spinage 2012). There are historical descriptions, for instance, of how these human migration patterns led to disease outbreaks in the new territories (e.g., influenza, smallpox, and measles). However, despite epidemiology having acknowledged the ecological aspects of infectious diseases since its start and invasion biology having some of its foundations in the spread and impacts of pathogens (e.g., Elton 1958 highlighted several examples of plant, animal, and human pathogens as biological invasions), the formal interaction between the two disciplines is quite recent and currently limited: The number of publications bridging the two disciplines is several orders of magnitude lower than in each field separately (figure 3).
Cumulative number of publications on biological invasions, human epidemics and the combination of the two topics according to the Web of Science from 1800 until 2020. Notice that the y-axis is in log scale. The search term for human epidemics was human epidemics whereas for biological invasions, the search term was ecological invasions. This term was more specific to retrieve all studies on that topic while excluding nontopic studies (e.g., cancer research, pharmacology and biomaterial science).
Approaches such as One Health, EcoHealth, Planetary Health, and One Biosecurity emphasize the links between human health, environmental health, and the health of plants and animals (Ogden et al. 2019, Hulme 2021). Following this principle, there have been recent attempts to cross-fertilize research on biological invasions and human infectious diseases from both conceptual and methodological perspectives. Although marked differences do exist in the ecology and evolution of human pathogens and free-living macroscopic invasive species, including issues of host specificity, immunity, and the temporal and spatial scales of interactions, opportunities exist to bring these disciplines together under a common framework (Lewis et al. 2016, Hulme et al. 2020). Previous reviews have mostly been focused on the stages of invasions and emerging infectious pathogens, especially those that also affect wildlife (Hatcher et al. 2012, Jeschke et al. 2013, Dunn and Hatcher 2015, Roy et al. 2017), on the role of invasive species as vectors or reservoirs of pathogens worldwide (Hulme 2014a, Rabitsch et al. 2017), or on spatial dynamics (Hulme et al. 2020). Most of these interdisciplinary approaches have been on particular taxa, habitats, or regions (Crowl et al. 2008, Medlock et al. 2012, Conn 2014). However, a detailed review of the parallels between scientific approaches to invasions and human epidemics is still missing.
Given increasing rates of emerging infectious pathogens and biological invasions worldwide and the ongoing global health crisis caused by the novel coronavirus SARS-CoV-2, the need for integrative and interdisciplinary approaches to biosecurity has never been greater (Nuñez et al. 2020, Pyšek et al. 2020, Hulme 2021). In the present article, we provide a holistic review of key parallels in the conceptual foundations in invasion biology and human infectious epidemics. Specifically, we describe approaches to the study of the pathways of introduction of invasive species and human pathogens, compare the stages and dynamics of the invasion process with those of epidemics, outline well-established hypotheses on the performance and impacts of invasive species and show their analogues in human pathogens, summarize the usefulness and limitations of forecasting tools, and finally discuss the implications for biosecurity.
Pathways of introduction of invasive species and transmission of pathogens
With globalization, the numbers of invasive species and human pathogens have increased exponentially in the twentieth century, with no sign of saturation (Jones et al. 2008, Seebens et al. 2017). Invasive species including pathogens are rapidly transported by the same global networks that move products and people to distant regions, where they are likely to encounter naive ecological and human communities that have not interacted with them before. For example, dengue virus, the causative agent of dengue fever, is expanding its distribution range, and it is now reported in 128 countries. The main factor of its spread is related to climatic change that benefits the Aedes aegypti mosquito, the main vector of the virus, and increased human movements between populations; even sporadic indigenous virus transmissions have occurred in previously dengue-free countries (Chomicz et al. 2016). Managing the pathways of introduction of invasive species and infectious pathogens is a prerequisite to implementing effective surveillance, early response, and mitigation policies (Essl et al. 2015, Ogden et al. 2019).
The Convention on Biological Diversity provides a global standard terminology for species introduction pathways that can be classified by six mechanisms: release, escape, transported as contaminant, transported as stowaway, corridors, and unaided (Saul et al. 2017). These can be further classified in 44 subcategories that identify their socioeconomic use and purpose of introduction (e.g., horticulture, pet trade, fisheries, game). Recently, this classification has been applied to thousands of non-native species introduced to Europe and worldwide (Pergl et al. 2020). Range expansion of native species that track environmental changes is an ecological phenomenon that gets often confounded with biological invasions. However, there are major functional, phylogenetic, physiological, behavioral, and phenological feature differences separating range-expanding from non-native species (Essl et al. 2019); accordingly, the two groups of species deserve to be treated as distinct biogeographic entities (Essl et al. 2020). Range-expanding species (i.e., neonatives) can also cause environmental and health impacts (Wallingford et al. 2020). However, to not increase the complexity of our review, we do not include range-expanding species in this study.
In human epidemiology, besides the dichotomy between active and passive introduction of pathogens (Mallon et al. 2015), a classification of pathways to such detail as in biological invasions is currently not available. The term pathways of introduction refers to the movement of the pathogen either as a free-living organism (environmental contamination) or via the original (reservoir) host, the vector, or human hosts. Infected hosts that travel with their newly acquired pathogens to distant places contribute to the pathogens’ geographical spread. Phylogenetic and genomic analyses are important tools used to reconstruct epidemiological origin, history, and links among infectious hosts. Genomic surveillance is not routinely used in biological invasions to identify the geographic origin and pathways of introduction of non-native macroorganisms (but see Hamelin and Roe 2020).
Transmission of emerging infectious pathogens can also be classified as zoonotic or nonzoonotic. A global analysis suggests that more than 60% of human emerging infectious pathogens are zoonotic, with 70% of these originating in wildlife (Jones et al. 2008). The IUCN list 100 of the World's Worst Invasive Alien Species contains 12 species that are reservoirs of pathogens that infect humans (table 1). The most well-known historical example is the house mouse (Mus musculus) and the black rat (Rattus rattus) as hosts of Yersinia pestis, causing bubonic plague. Other invasive species include the small Indian mongoose (Herpestes javaricus) and the crab eating macaque (Macaca fascicularis) as reservoirs for rabies. Zoonoses, by definition, involve pathogen spillover from a vertebrate host to humans, although subsequent human-to-human transmission is sometimes possible. These host-switching events from wildlife reservoir to human can be preceded by an invasion event—for example, when the reservoir host enters a previously unoccupied area (e.g., wildlife transported to an urban market) or followed by an invasion event (e.g., when infected people travel, with their newly acquired pathogens, to distant places). Zoonotic spillover is seen for multiple pathogens including Plasmodium species (causative agent of malaria), Trypanosoma brucei (trypanosomiasis), a Leishmania species (leishmaniasis), influenza A (flu), human immune deficiency virus (AIDS), ebolavirus (Ebola hemorrhagic disease), as well as the new coronavirus related to MERS-CoV and SARS-CoV (Karesh et al. 2012).
In invasion biology, prevention requires an analysis of how the invasive species likely will arrive to a new region (primary introduction) and how it spreads subsequently in the surrounding region (secondary spread). This dual pathway classification has seldom been applied in emerging infectious pathogens despite that it is well known that socioeconomic variables (e.g., behavior, income, tourism, military deployment, trade) can highly influence transmission. An improved understanding of mechanisms that link long- and short-distance pathogen spread with the socioeconomic characteristics of the hosts is essential to prevent and manage epidemics.
Stages and dynamics of invasions and epidemics
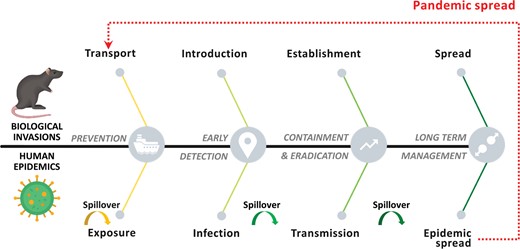
There are several distinct terms used to describe processes of invasion and those of an epidemic, but conceptually, the invasion of ecosystems and the infection process at the individual and population level follow essentially the same basic series of stages—that is, transport or exposure, introduction or infection, establishment or transmission, and spread or epidemics, respectively (Jeschke et al. 2013, Dunn and Hatcher 2015, Plowright et al. 2017, Hulme et al. 2020, Nuñez et al. 2020). In both cases, whether a particular invasive species or pathogen is able to pass on to the next stage and has consequences for the receiving ecosystem or host depends on many filters and can be substantially influenced by human interventions (figure 4). These stages have used different terminology for invasions and infections, respectively, as is indicated below.
Comparing the stages of biological invasions and human epidemic (adapted from Woolhouse and Gaunt 2007, Blackburn et al. 2011, Hatcher et al. 2012, Jeschke et al. 2013), and possible management actions at these stages (adapted from Dunn and Hatcher 2015, Robertson et al. 2020). Pathogens that emerge and cause an epidemic anywhere on the globe can be transported and spread globally leading to a pandemic in the worst case (the dotted arrow). The bent arrows indicate potential positions of zoonotic pathogen interspecific spillover.
Transport or exposure. International transport of the non-native species by human agency is the first stage of the biological invasion process. Similarly, in emerging infectious pathogens, the international movement of hosts (e.g., planes or boats) represents the first contact (or exposure) of humans with infected human hosts. The pathogen may originate in wildlife or domestic vertebrates and spillover to humans either through a vector (e.g., insects) or through direct contact (i.e., zoonosis).
Introduction or infection. Following transport, some non-native species are released directly into the wild (e.g., for fishing or hunting purposes), escape from captivity (e.g., pets) or cultivation (e.g., ornamental plants), or move unaided utilizing artificial corridors (e.g., waterways). A pathogen can also be introduced through released and escaped reservoirs or move unaided through air (e.g., air conditioning) or water (e.g., sewage) infrastructures. For a pathogen, at the individual host level, this is the infection stage, during which it enters the host body, circumventing behavioral, physical, and physiological barriers. Many human infectious pathogens such as Hendra virus, West Nile virus, or the strain of influenza A causing avian flu result from independent spillover from reservoirs with little human-to-human transmission. These outbreaks tend to be short lived but, nonetheless, can have high impact in humans (e.g., the case fatality rate for some avian flu is 60%; Greger 2007).
Establishment or transmission. The establishment of an invasive species is the process by which a founding non-native population reproduces, increases in size, and becomes self-sustaining in the new range. Invasive species introduced to a new region have to overcome several biotic and environmental barriers imposed by the recipient region and its biota (Blackburn et al. 2011). For a pathogen, at the level of the individual host, this is equivalent to overcoming immunological barriers that allow within-host persistence, multiplication, and transmission to new hosts. Widespread transmission and establishment within a new host population occurs when the basic rate of reproduction (R0, the number of secondary cases resulting from each primary case) exceeds 1. The likelihood of the pathogen evolving to become self-sustaining in the human population increases with the spillover rate, the current R0, and the mutation rate (Antia et al. 2003). For example, during the 2013–2016 Ebola virus outbreak, three adaptive mutations in the virus genome occurred that affected the functional activity of various viral proteins increasing its ability to enter human cells, grow, and be transmitted (Urbanowicz et al. 2016).
Spread. Finally, spread is the process by which an invasive species expands its range in the introduced region beyond the area or host population in which it was first established. This matches with the definition of epidemics as the spread of the pathogen to many people in a locality during a short period. Such an expansion of a pathogen in a human population can occur through increased animal-to-human contacts (spillover) or through human-to-human transmission. For human infectious pathogens, spread can occur anywhere along a gradient from transmission between individuals in a local population, to global transport of infections between populations. Like biological invasions in general, the large scale spread of pathogens follows hub-and-spoke network dynamics and does not occur homogeneously but, rather, in discrete, sometimes lengthy jumps, facilitated by human transportation systems such as air travel (Strickland et al. 2015). The most serious outcome of an emerging pathogen is a pandemic—an epidemic occurring worldwide or over a very wide area, crossing international boundaries, and usually affecting a large number of people.
Unprecedented opportunities for pathogen spread and transmission are generated by technological advances and social activities driving human mobility, as is evident in the movement of millions of humans between continents on a daily basis (Tatem et al. 2006) and with increasingly crowded living conditions and inadequate access to water, sanitation, and health care in many areas of the world. For example, the first cases of SARS-CoV-2 in many countries were associated to business and tourism, whereas subsequent local spread was mainly related to factors such as housing density and occupational exposure (Bassino and Ladmiral 2020). Owing to global transportation networks, introduced organisms—both pathogens and free-living macroscopic species—create satellite outbreaks in distant regions that contribute to exponential rates of spatial expansion.
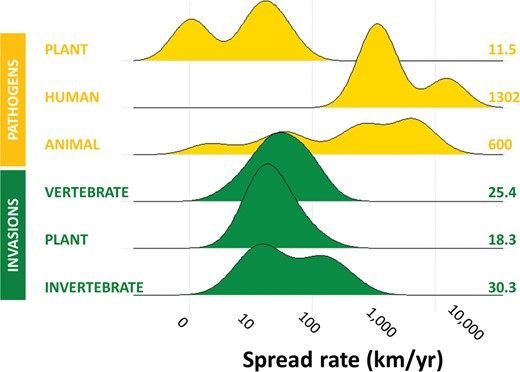
Rate of spread. There are temporal and spatial differences in the dynamics of epidemics and invasions. In an epidemic, the speed by which the pathogen can spread is usually faster than the invasion of a free-living macroscopic species (Peterson 2008). The spread of human epidemic pathogens can be explosive. It is generally one to three orders of magnitude faster than for invasive species and plant pathogens (figure 5). This is because of their short generation times, high mutational rate, and effective population sizes that are orders of magnitude higher. The rates of spread of terrestrial flora and fauna are typically in the range of 0.1–100 kilometers (km) per year (Hulme 2014b, Horvitz et al. 2017) with mobile species such as many invertebrates (e.g., forest pest insects) being faster (Roques et al. 2016). In contrast, human epidemic viruses such as Zika, Ebola, and West Nile virus, can spread at rates of 103–104 km per year (Zinszer et al. 2015, 2017, Hadfield et al. 2019), a velocity only reached in some pathogens of marine wildlife (McCallum et al. 2003).
Density plot showing the frequency of observed radial spread rates (log scale) for different pathogens and invasive taxonomic groups. The height of each density curve indicates the relative number of data points, normalized to 1. The numbers at the right indicate the median rate of spread for the group. The figure was created with packages ggplot2 and ggridges in R v. 4.0.0. The raw data were extracted from Smal and Fairley (1984), van den Bosch and colleagues (1992), Holmes (1993), Teangana and colleagues (2000), McCallum and colleagues (2003), Phillips and colleagues (2007), Pioz and colleagues (2011), Fraser and colleagues (2015), Zinszer and colleagues (2015, 2017), Evans (2016), Roques and colleagues (2016), Horvitz and colleagues (2017), and Hadfield and colleagues (2019).
These differences in spread velocity matter because they influence the response of the recipient systems in many ways. For instance, rapid range expansion could render phenotypic or genotypic adjustments in recipient populations and communities less likely. Moreover, success in the control of invasive species and infectious pathogen spread is highly dependent on the spatial distribution of introductions (Hulme et al. 2020). The scattered nascent foci of invasive species or infested hosts have the potential to spread more rapidly than one large continuous focus (Moody and Mack 1988). The recommendation to detect, isolate, and trace every contact of the SARS-CoV-2 infected individual follows this principle (e.g., Pagliari 2020).
Lag times. This phenomenon has received a fair amount of attention in invasion biology to define the duration between invasion stages and also between the introduction and the onset of rapid range expansion (Crooks 2005, Rouget et al. 2016, Spear et al. 2021). Lag times are particularly evident in ornamental plant species that only start to spread several decades after being introduced (Kowarik 1995). Many populations of non-native plants are dependent on repeated introductions and need a long residence time before they form self-sustaining, viable populations (Dlugosch and Parker 2008). Small populations are very sensitive to environmental stochasticity that might limit their survival, reproduction, and dispersal during early stages of invasion (Mack 2000). There are many cases of non-native species that were unnoticed for a long time and only became invasive as a response to environmental changes.
Lag times are also identified in emerging human pathogens, owing to the latency period between infection and disease symptoms that can range from a few days (e.g., SARS-CoV) to years (e.g., HIV). More precise time intervals than for invasions are defined for pathogens in terms of stages of the pathogen life cycle and disease symptoms (Bar-On et al. 2020). For example, in virus infections, time lags within an individual host are decomposed into the eclipse period (the time to make intracellular virions), the latent period (the time from cell entry until the appearance of the first extracellular viruses), the infectious period (from infection to transmission), and the incubation period (from infection to the emergence of symptoms). The lengths of these four periods are of paramount importance to slow down and deter the transmission stage to an epidemic spread by establishing quarantine and confinement periods.
Many invasive species that are vectors of human parasites are increasing their ranges because of global warming (Medlock and Leach 2015). Similarly, many infectious diseases are increasing with climate change (e.g., by speeding up the life cycle of the pathogens). For example, human and dog infections by Dirofilaria nematodes are becoming more frequent in Northern Europe with increasing summer warming that facilitates parasite incubation (Genchi et al. 2011). The recognition of long lag times and the role of environmental changes in invader and parasite dynamics suggests that we need to endorse the precautionary principle: one should assume that any invader or pathogen has the potential for undesirable effects and that lengthy periods of seemingly innocuous behavior can be a poor predictor of how these organisms will behave in the future (Crooks 2005).
Hypotheses explaining biological invasions and analogues to epidemics
Invasion biology has formulated and tested several hypotheses on why some non-native species go through the stages of the invasion process, whereas others do not (e.g., Catford et al. 2009, Jeschke and Heger 2018). Invasions are influenced by many factors, and these can be grouped into five categories related to propagule pressure, organism traits, biotic interactions, eco-evolutionary experience, and recipient system characteristics (Enders et al. 2020). Each of these five categories encapsulates several hypotheses reviewed by Jeschke and colleagues (2020) and provides a different perspective on the causes of invasion. In the present article, we explore the potential parallels between biological invasions and human epidemics across the five categories of hypotheses. A detailed dissection of them is presented in the supplemental material.
Propagule pressure. Propagule pressure refers to the frequency and size (i.e., numbers of propagules introduced) of introduction events (Lockwood et al. 2005). A non-native species is more likely to become invasive in a given region if it is introduced multiple times and with higher numbers of individuals. This hypothesis is also applicable to human pathogens both from an individual and a population perspective and at all stages of the infection process. Pathogen pressure is defined as the abundance of pathogens exposed to the human host at a given point in space and time. With increasing pathogen pressure, there is an increasing likelihood that the pathogen will establish and undergo exponential growth within an individual host, reflecting the well-known dose–response curve (Horrocks et al. 2011). The same idea applies to the population level; it is well known that the number of infected individuals entering a population can strongly influence pathogen dynamics (Ostfeld et al. 2008), as can the heterogeneity of pathogen transmission by individuals (Woolhouse et al. 1997), such as the presence of superspreaders (Lloyd-Smith et al. 2005). That is, the greater the number of infectious (reservoir or human) hosts to arrive in a given locality, the higher the likelihood that the pathogen will establish and spread in the population (Correa-Martínez et al. 2020). This concept of pathogen pressure is also useful to understand the spillover stage in zoonotic diseases. Pathogen pressure depends on the pathogen dynamics in reservoir hosts, the pathogen's release from reservoir hosts, and the pathogen's survival or dispersal outside of reservoir hosts (Plowright et al. 2017).
Organism traits. Some traits—mainly related to growth, reproduction, and dispersal rates—explain why some non-native species have higher invasiveness (i.e., intrinsic potential to become invasive). For example, pine species with small seeds and short generation time have higher potential to invade (Richardson and Rejmánek 2011). Likewise, animals such as rats and pigeons are notorious invasive species worldwide and have key characteristics that form the basis of their establishment to new areas (e.g., they are generalists, have high plasticity to cope with different environmental conditions, and have adapted to urban environments). Some invasive species that are reservoirs or vectors of human parasites also share some of these traits: a young age at maturity, large and frequent broods, an explosive rate of replication, tolerance to harsh environmental conditions including disturbances, high mobility during at least one life stage, and strategies of abundant dispersal (Ostfeld et al. 2014).
Similarly to those of invasive species, different life-history traits of human pathogens appear related to the pathogens’ ability to establish persistent infections within individual hosts and their transmission from host to host. Two key traits that affect pathogen fitness are virulence and transmissibility. They are related to, for example, their capacity to invade cells by adhering to specific receptors, the production of exoenzymes and toxins that allow them to colonize specific tissues of the hosts, and their capability to evade the immune system by self-protecting from phagocytosis, exploiting molecules produced by the host or by antigenic variation (Alcami and Koszinowski 2000). Antigenic variation, the production of different variants of a protein implicated in the interactions with the host cells (Palmer et al. 2016) is a similar strategy as the phenotypic variation of invasive species to cope with different environmental conditions (Davidson et al. 2011). Host specificity is another trait that influences pathogen fitness and epidemics. Generalist pathogens, those that can survive in different hosts, are more likely to cause zoonotic spillover (Woolhouse 2002). These pathogens tend to use cell receptors, which are conserved across different host species (Parrish et al. 2008).
Rapid evolution can lead to increased invasiveness of non-native species and to higher virulence and transmissibility of pathogens, either native or non-native. Evolutionary changes during the time span of a few centuries can allow plant physiology to adapt to the new climatic conditions of the introduced range (Maron et al. 2007). Similarly, evolved resistance to pesticides also explains high infestation levels of weeds and pests in crops. In humans, the massive use of antibiotic treatments is causing the emergence of novel, resistant bacteria strains. For example, antibiotic resistance is increasing sexually transmitted diseases such as Neisseria gonorrhoeae and Haemophilus ducreyi, the causative agents of gonorrhea and chancroid, respectively (Ison et al. 1998, González-Candelas et al. 2019).
Biotic interactions. Interactions between non-native and native species are crucial for understanding invasions. A key point in this discussion is that the same non-native species can establish in one ecosystem and not in another, depending on local biotic interactions (Zenni and Nuñez 2013). Probably the most popular example on how biotic interactions shape the invasion process is the enemy release hypothesis, which posits that the absence of enemies in the introduced range is a cause of invasion because introduced species left their pathogens, parasites, and predators behind when colonizing a new ecosystem (Maron and Vilà 2001, Keane and Crawley 2002). The natural enemies for pathogens are virophages and bacteriophages of the human microbiota (Dalmasso et al. 2014). Most probably, in zoonoses, when pathogens jump from their original animal host to a human host, virophages and bacteriophages in humans do not identify and act against the new pathogen.
Mutualistic interactions between invasive and native species (e.g., animal mediated pollination, seed dispersal, and symbioses between plant roots and microbiota) can be disruptive for the native species but highly beneficial to the integration of the invasive species in the recipient ecosystem (Richardson et al. 2000). A similar situation in emerging pathogens is the case of coinfections among pathogen or parasite species or strains or clones of the same species. A clear case is HIV, which makes the host susceptible to a range of other pathogens. The outcome of biotic interactions can be antagonistic (competition and superparasitism), neutral, but also mutualistic (Griffiths et al. 2011). These interactions have significant epidemiological clinical and evolutionary implications because they affect the susceptibility of the host to subsequent infections as well as pathogen virulence and transmissibility. For example, given the trade-off between type 1 and type 2 immune responses induced by micro and macroparasites, coinfection with endemic helminth infections has been predicted to increase the severity of SARS-CoV-2 (Bradbury et al. 2020). Even if pathogens do not interact, the death of coinfected hosts can decrease the fitness of the individual pathogens (Hamelin et al. 2019).
Eco-evolutionary experience. A long-standing hypothesis explaining the impact of biological invasions is that species introduced to ecosystems lacking functionally or phylogenetically similar natives are more likely to disrupt communities, because these communities lack effective physiological, morphological, or behavioral adaptations; that is, they are naive to such invasive species (Diamond 1986, Ricciardi and Atkinson 2004). Eco-evolutionary naïveté explains why native prey populations typically suffer greater damage from introduced consumers than from native consumers (Salo et al. 2007, Paolucci et al. 2013, Saul and Jeschke 2015, Anton et al. 2020). The hypothesis also predicts heightened sensitivity of insular ecosystems, such as islands and lakes, to the effects of invasions. For example, oceanic island endemisms have been devastated by non-native mammalian predators and herbivores, largely because most island biota evolved in the absence of such species (Russell et al. 2017). The eco-evolutionary experience hypothesis also applies to sessile organisms such as plants (Mack 2003). A novel plant life form in a new range can affect its invasiveness as well as the magnitude of its impact on native vegetation. For example, pines originated in the Northern hemisphere, and their impacts are larger when introduced in the Southern hemisphere, where not only the taxon but also the life form is completely new in many communities it invades. Differences in the mechanisms of pine impacts among regions are not well known but might be related to different biogeochemical effects on the soil to which the native plants are not adapted (Davis et al. 2019).
Analogously, immunological naïveté to infectious agents contributes to a large public health toll. Historical exposure and coevolution between hosts and pathogens typically lowers its severity within a population or region. In the case of malaria, for example, human populations at higher altitudes in the East African highlands are more susceptible to infection and suffer more severe symptoms compared with populations in lower-latitude areas, where they have had greater and longer exposure to the parasite (Pascual et al. 2008). Paralleling invader–community interactions, the more experienced hosts within pathogen–host interactions offer resistance to infection and experience less harm (Domínguez-Andrés and Netea 2019). Influenza pandemics, for example, cause lower mortality in populations that have had some evolutionary exposure (immunological memory) from previous pandemics (Horimoto and Kawaoka 2005). However, pandemics typically involve novel viruses arising from antigenic shift or zoonotic spillover, which preclude human populations from having immunity. For example, the emergence of swine flu in 2009 resulted from recombination of segments of influenza A from pigs, birds, and human hosts, creating a strain with the ability to target human respiratory receptors but with a novel antigenic profile (Smith et al. 2009). Within a human population, naïveté decreases as more people are infected. Once some immunity develops within the host population, the Reff (effective reproduction number) will decline, a phenomenon that is exploited in the use of vaccination programs.
Recipient system characteristics. Pristine native ecosystems with high biodiversity often resist invasion via a process termed biotic resistance (Levine and D'Antonio 1999). Similarly, ecosystems with high animal and plant diversity have consistently been shown to reduce the transmission of infectious pathogens because of reduced chances to encounter hosts (Keesing et al. 2010, Myers et al. 2013, Johnson et al. 2015). In the case of pathogens, the limitation in the establishment of a new microorganism when the invaded community has high species diversity is rooted on the microbiostasis concept (Mallon et al. 2015). Plant and microbe experiments using synthetic communities from low to high diversity species assemblages have shown that invader establishment and abundance increase in depauperate communities (Zavaleta and Hulvey 2004, Eisenhauer et al. 2013). In humans, the microbiome is a barrier to pathogens (Penders et al. 2013). The relationship between alterations of the microbiome composition and diversity with antimicrobial resistance is a topic of major research interest in biomedicine.
The diversity–invasion relationship can be uncoupled with increased availability of resources. Disturbances offer windows of opportunity for invasive species by disrupting biotic resistance and freeing resources (Hobbs and Huenneke 1992, Jeschke and Heger 2018). Disturbances can also preadapt plants and animals for colonization of human-dominated ecosystems (Hufbauer et al. 2012). The same appears to be true for epidemics. After natural disasters, there are numerous opportunities for pathogen outbreaks driven by people crowding, poor sanitation leading to increased exposure to pathogens, and malnutrition increasing susceptibility to disease (Watson et al. 2007). Altered ecosystems by deforestation, agricultural expansion, harvesting of bush meat, and other anthropogenic disturbances can facilitate the emergence of zoonotic pathogens (Keesing et al. 2010) and create opportunities for spillover (Jones et al. 2013). For example, in Australia and Asia, changes in land use and habitat loss have changed the ecology and behavior of fruit bats that are natural reservoirs of the Nipah and Hendra viruses, increasing spillover chances to humans (Kessler et al. 2018). At the level of the individual host, altered immunological or physiological conditions affect susceptibility to infection and the severity of the disease (Plowright et al. 2017). For example, certain medicines, immunosuppression caused by coinfections or medical or surgical procedures, nutrition, and autoimmune diseases offer windows of opportunity for infection.
In summary, the invasions and epidemics are driven by historical, intrinsic, and extrinsic characteristics of the species or pathogens, such as the abundance of propagules, the frequency of the introduction events, the attributes of interacting species or strains, and the characteristics of the invaded or host system. The interplay and importance of these factors are highly context specific and highly dependent on the spatial scale of analysis (von Holle and Simberloff 2005, DeVincenzo et al. 2010).
Forecasting biological invasions and human epidemics
Forecasting the occurrence and timing of future invasions is challenging, owing to the high intrinsic uncertainty associated with many potential origins, trends, and pathways of introduction, particularly for new invasive species that have not been previously recorded as problematic (Seebens et al. 2018). Similar challenges apply to emerging human pathogens. The analysis of past events has facilitated the identification of potential spatiotemporal patterns of invasion and pathogen emergence, which allows prioritizing surveillance efforts on the most likely threats and vulnerable areas. For instance, invasive species are dominated by plants (e.g., lantana, kudzu, water hyacinth), they are dispersed by human activities that involve transportation and commerce, and their global spread is largely driven by climate, land use, and environmental degradation (Pyšek et al. 2020). Likewise, most pandemics (e.g., HIV, severe acute respiratory syndrome, COVID-19) appear to have originated in animals, they are caused by viruses, and their emergence is driven by ecological, behavioral, or socioeconomic changes (Morse et al. 2012). For example, a study in 2013 reported the presence of a large reservoir of SARS-like coronaviruses in horseshoe bats, which, together with the custom of eating non-native mammals in southern China, was already alerting epidemiologists to the risk of a human epidemic (Ge et al. 2013). Some of the differences and common challenges shared between the study of biological invasions and emerging pathogens are outlined below and summarized in table 2.
Differences and common challenges associated with the forecasting of biological invasions and human epidemics with indications of the potential for collaboration and cross-fertilization across disciplines.
| . | Biological invasions . | Human epidemics . | Potential cross-fertilization across disciplines . |
|---|---|---|---|
| Data used | Geo-referenced species occurrence | Number of infected individuals | Common monitoring systems and data platforms |
| Rarely, abundance data | Information rarely geo-referenced | ||
| Indicators (developed to follow an outbreak) | Likelihood of species presence (suitability) | R0, likelihood of exponential spread | Correlation between disease and invasion indicators |
| Number of non-native species | |||
| Models | Mostly spatially, niche-based (e.g., species distribution models) | Dynamic, biology-based e.g., Susceptible Immune Recovered (SIR) | Sharing modeling tools and advances to reduce uncertainty |
| Scales | Regional to global | Local to regional | Automatically updated platforms to follow an outbreak |
| Years to decades | Rarely global | ||
| Weeks to months | |||
| Critical factors (ordered) | Climate | Biological (e.g., transmissibility) | Share environmental and human data for modeling |
| Environmental conditions | Human activities (e.g., transport) | New sources of human-related data (e.g., mobile phones, trade flows) | |
| Human activities (e.g., transport, land use) | Human behavior (e.g., sociability) | ||
| Biological (e.g., dispersal) | Management (e.g., medical and nonmedical actions) | ||
| Approaches | Exploratory | Intervention scenarios | Common scenario frameworks and workflows |
| Climate change scenarios | |||
| Management scenarios | |||
| Common challenges | Data quality and quantity | ||
| Modeling of complex systems under imperfect detection | |||
| Incorporating human activities and behaviors | |||
| Anticipating alternative policy and management scenarios | |||
| High intrinsic uncertainty associated to exponential processes | |||
| Traceability of origin and expansion of pathogen or invader | |||
| Lag phases (e.g., between introduction and impact, between management and effective mitigation) | |||
| Anticipating the next biological threat based on transmissibility or spread and potential impacts |
| . | Biological invasions . | Human epidemics . | Potential cross-fertilization across disciplines . |
|---|---|---|---|
| Data used | Geo-referenced species occurrence | Number of infected individuals | Common monitoring systems and data platforms |
| Rarely, abundance data | Information rarely geo-referenced | ||
| Indicators (developed to follow an outbreak) | Likelihood of species presence (suitability) | R0, likelihood of exponential spread | Correlation between disease and invasion indicators |
| Number of non-native species | |||
| Models | Mostly spatially, niche-based (e.g., species distribution models) | Dynamic, biology-based e.g., Susceptible Immune Recovered (SIR) | Sharing modeling tools and advances to reduce uncertainty |
| Scales | Regional to global | Local to regional | Automatically updated platforms to follow an outbreak |
| Years to decades | Rarely global | ||
| Weeks to months | |||
| Critical factors (ordered) | Climate | Biological (e.g., transmissibility) | Share environmental and human data for modeling |
| Environmental conditions | Human activities (e.g., transport) | New sources of human-related data (e.g., mobile phones, trade flows) | |
| Human activities (e.g., transport, land use) | Human behavior (e.g., sociability) | ||
| Biological (e.g., dispersal) | Management (e.g., medical and nonmedical actions) | ||
| Approaches | Exploratory | Intervention scenarios | Common scenario frameworks and workflows |
| Climate change scenarios | |||
| Management scenarios | |||
| Common challenges | Data quality and quantity | ||
| Modeling of complex systems under imperfect detection | |||
| Incorporating human activities and behaviors | |||
| Anticipating alternative policy and management scenarios | |||
| High intrinsic uncertainty associated to exponential processes | |||
| Traceability of origin and expansion of pathogen or invader | |||
| Lag phases (e.g., between introduction and impact, between management and effective mitigation) | |||
| Anticipating the next biological threat based on transmissibility or spread and potential impacts |
Differences and common challenges associated with the forecasting of biological invasions and human epidemics with indications of the potential for collaboration and cross-fertilization across disciplines.
| . | Biological invasions . | Human epidemics . | Potential cross-fertilization across disciplines . |
|---|---|---|---|
| Data used | Geo-referenced species occurrence | Number of infected individuals | Common monitoring systems and data platforms |
| Rarely, abundance data | Information rarely geo-referenced | ||
| Indicators (developed to follow an outbreak) | Likelihood of species presence (suitability) | R0, likelihood of exponential spread | Correlation between disease and invasion indicators |
| Number of non-native species | |||
| Models | Mostly spatially, niche-based (e.g., species distribution models) | Dynamic, biology-based e.g., Susceptible Immune Recovered (SIR) | Sharing modeling tools and advances to reduce uncertainty |
| Scales | Regional to global | Local to regional | Automatically updated platforms to follow an outbreak |
| Years to decades | Rarely global | ||
| Weeks to months | |||
| Critical factors (ordered) | Climate | Biological (e.g., transmissibility) | Share environmental and human data for modeling |
| Environmental conditions | Human activities (e.g., transport) | New sources of human-related data (e.g., mobile phones, trade flows) | |
| Human activities (e.g., transport, land use) | Human behavior (e.g., sociability) | ||
| Biological (e.g., dispersal) | Management (e.g., medical and nonmedical actions) | ||
| Approaches | Exploratory | Intervention scenarios | Common scenario frameworks and workflows |
| Climate change scenarios | |||
| Management scenarios | |||
| Common challenges | Data quality and quantity | ||
| Modeling of complex systems under imperfect detection | |||
| Incorporating human activities and behaviors | |||
| Anticipating alternative policy and management scenarios | |||
| High intrinsic uncertainty associated to exponential processes | |||
| Traceability of origin and expansion of pathogen or invader | |||
| Lag phases (e.g., between introduction and impact, between management and effective mitigation) | |||
| Anticipating the next biological threat based on transmissibility or spread and potential impacts |
| . | Biological invasions . | Human epidemics . | Potential cross-fertilization across disciplines . |
|---|---|---|---|
| Data used | Geo-referenced species occurrence | Number of infected individuals | Common monitoring systems and data platforms |
| Rarely, abundance data | Information rarely geo-referenced | ||
| Indicators (developed to follow an outbreak) | Likelihood of species presence (suitability) | R0, likelihood of exponential spread | Correlation between disease and invasion indicators |
| Number of non-native species | |||
| Models | Mostly spatially, niche-based (e.g., species distribution models) | Dynamic, biology-based e.g., Susceptible Immune Recovered (SIR) | Sharing modeling tools and advances to reduce uncertainty |
| Scales | Regional to global | Local to regional | Automatically updated platforms to follow an outbreak |
| Years to decades | Rarely global | ||
| Weeks to months | |||
| Critical factors (ordered) | Climate | Biological (e.g., transmissibility) | Share environmental and human data for modeling |
| Environmental conditions | Human activities (e.g., transport) | New sources of human-related data (e.g., mobile phones, trade flows) | |
| Human activities (e.g., transport, land use) | Human behavior (e.g., sociability) | ||
| Biological (e.g., dispersal) | Management (e.g., medical and nonmedical actions) | ||
| Approaches | Exploratory | Intervention scenarios | Common scenario frameworks and workflows |
| Climate change scenarios | |||
| Management scenarios | |||
| Common challenges | Data quality and quantity | ||
| Modeling of complex systems under imperfect detection | |||
| Incorporating human activities and behaviors | |||
| Anticipating alternative policy and management scenarios | |||
| High intrinsic uncertainty associated to exponential processes | |||
| Traceability of origin and expansion of pathogen or invader | |||
| Lag phases (e.g., between introduction and impact, between management and effective mitigation) | |||
| Anticipating the next biological threat based on transmissibility or spread and potential impacts |
Data. Problems of low data quality and uneven sampling effort are common for both fields. Data on species occurrence, used in invasion studies, is strongly biased geographically and taxonomically (Pyšek et al. 2008), with invasive pathogens being specially understudied (Roy et al. 2017). Similarly, in an epidemic the quality of data on the number of infections, deaths, tests, and other factors needed for robust modeling is often limited by underdetection, reporting delays, and poor documentation (Jewell et al. 2020). Recent methods for estimating occupancy dynamics under imperfect detection are promising to reduce the uncertainty of predictions, particularly for host–pathogen systems (Bailey et al. 2014). The two fields would benefit from common monitoring systems and open data platforms to facilitate standardization and data sharing.
Indicators. The focus of invasive species forecasts is usually the likelihood of species presence or absence and, therefore, the total number of invasive species that could invade an area, rather than their potential abundance or impacts. In contrast, the most important indicator used to assess the spread rate of an epidemic is R0. The larger the value of R0, the harder it is to control an epidemic. The demographic analogue for invasive species is lambda (λ), the population rate of change (Caswell 2000). When applied to population dynamics, a value of λ < 1 will similarly lead to population decline and, ultimately, extinction. In both cases, however, any value that is even only slightly above 1 will lead to population growth of the invasive species or pathogen, until other limiting factors set in. Calculating λ for invasive species is knowledge and data intensive and becomes complicated because individuals can reproduce and disperse for many years, and survival depends on multiple factors that can be deeply affected by environmental gradients (Krkosek and Lewis 2010). This has limited the use of population models to rather few invasive species with enough information, frequently plants and invertebrates (Buchadas et al. 2017). Considering the close relationship between biological invasions and epidemics, the use of common spatiotemporal indicators of risk would provide insights into their interrelationship and common underlying drivers (Allen et al. 2017, Hulme et al. 2020).
Models. Among the multiple modeling techniques employed in invasion studies, species distribution models have become the gold standard method to identify the habitats or geographical areas most prone to be invaded under current and future climate change scenarios (e.g., Thuiller et al. 2005, Bradley 2010). In contrast, from the 174 infectious pathogens with comprehensive geographical information, only 7 (4%) had been comprehensively mapped including dengue, Lassa, Mayaro, monkey poxviruses, and the malaria parasites Plasmodium falciparum and Plasmodium vivax (see Hay et al. 2013). This is likely because of the complex characteristics of the host–pathogen system, which requires a reevaluation of the traditional biogeography framework (sensu pathogeography in Murray et al. 2018). In this sense, a key difference between invasive species and epidemics originated by pathogens with complex life cycles is that the distribution of the pathogen is defined by the joint distributions of all species involved in its transmission cycle as dictated by the suitable ecological conditions and dispersal limitations for each. Consequently, models should integrate the large biogeographic factors that condition the presence of vectors, hosts, and reservoirs, with the microscale characteristics of hosts that allow the survival, reproduction, and transmission of pathogens (Johnson et al. 2019). Multispecies joint distribution modeling (Pollock et al. 2014) could be interesting for infectious diseases, particularly for multihost pathogens or to investigate the interaction among pathogens. Furthermore, a better understanding of the global distribution of mammal zoonotic hosts, including invasive animals, could help predict future hotspots of zoonotic pathogen emergence (Han et al. 2016).
However, not all pathogens are appropriate for species distribution modeling, depending on their life cycle, hosts, and spread mode. Instead, dynamic models explicitly represent the key population groups and central processes of epidemic spread. Dynamic models can be used to predict future trends of pathogen spread, although the uncertainty of exponential processes such as epidemics is considerable. Dynamic models have been increasingly used for invasive species since the late 1990s, mostly focused on plants such as the blue-leafed wattle (Acacia saligna) and on invertebrates like the zebra mussel (Dreissena polymorpha; see Buchadas et al. 2017 for a review). Dynamic models are especially useful to support local management of invasions, but they are not routinely implemented, probably because of the high data demand, complex model procedures, and detailed parameterization needed to understand, analyze, and forecast biological invasions (Gallien et al. 2010). Hybrid models that combine the low data requirements of statistical models (such as species distribution models) with the ability of dynamic models to describe underlying processes are promising to improve the reliability of forecasts and facilitate the optimization of management and governance (Gallien et al. 2010). In the fundamental susceptible–infected–recovered model, groups of individuals within the host population are classified as “susceptible” to infection, “infectious” and able to transmit the pathogen, or “recovered” and immune to reinfection (Lloyd-Smith et al. 2009). Recently, the Epidemiological Framework for Biological Invasions (EFBI) has adapted susceptible–infected–recovered compartment models to characterize biological invasions by treating ecosystems as hosts and has allowed generalizations from epidemiology, such as the force of infection, the basic reproductive ratio R0, superspreaders, herd immunity, cordon sanitaire, and ring vaccination, to be discussed in the novel context of non-native species (Hulme et al. 2020).
Factors. Environmental conditions, including climate, set the minimum requirements necessary for survival but rarely prevent the distribution of either invasive species or human pathogens (Ibáñez et al. 2006). Beyond climate, invasive species modeling has demonstrated that accounting for human related factors associated with the pathways of introduction and propagule pressure, such as human population density, transportation networks, and anthropogenic degradation, is critical to increase the reliability of predictions (Gallardo et al. 2015). The same can be expected for the modeling of infectious pathogens that use information on human population density and movement to improve forecasts (e.g., Colizza et al. 2006, Tatem et al. 2006). Incorporating human behavior, education, and culture into models remains challenging for both disciplines but could be facilitated by nontraditional sources of information, such as mobile apps, news media, citizen science, social media, or syndromic surveillance.
Approaches. Studies of biological invasions are often used to anticipate the number and spatial coverage of invasions under current and future scenarios. In contrast, epidemiologic models are frequently used to estimate the relative effect of medical (e.g., vaccination) and nonmedical (e.g., social distancing, use of masks) interventions in reducing risk. For instance, the University of Oxford and Imperial College both provided intervention scenarios for SARS-CoV-2 pandemic that allowed the calculation of the estimated effect of various combinations of COVID-19 countermeasures on R0 (https://bit.ly/3ezKciZ; Ferguson et al. 2020). Intervention scenarios on the impact of biological invasions are less developed (but see Lenzner et al. 2019) and could greatly benefit from this approach.
Biosecurity
Although they are based on quite different disciplines, the fields of public health and invasion biology share similar goals in terms of having to deliver procedures and policies that lead to the exclusion, eradication, or effective management of biological risks. Biosecurity policies should, by definition, encompass the risk both to human health and to the environment arising from the emergence of pathogens and invasive species. However, in practice nation states and multilateral conventions address these risk through quite different mechanisms (Hulme 2011). Nevertheless, many biosecurity risks transcend the traditional boundaries of human health and the environment and call for a unified framework to reduce these risks (Hulme 2020). For example, the two most common invasive non-native rats worldwide are the black rat R. rattus and the brown rat Rattus norvegicus. Rat-borne pathogens have claimed more human lives than all the wars in history combined (Hulme 2014b). The omnivorous feeding habits of rats are also implicated in crop losses and in causing the decline of many small mammals, birds, reptiles, and invertebrates. Their effect has been particularly severe on islands, where rats have had more impact on endemic biodiversity than any other factor (Towns et al. 2006). Furthermore, the global drivers of future risks to public health and the environment from emerging human pathogens and invasive species share many parallels. For example, climate change is likely to facilitate the poleward expansion of human pathogens and non-native species, greater urbanization will lead to new hotspots for novel human pathogens and invasive species, the growth in international travel has been a major pathway for infectious diseases and non-native species, and increased intensification of agriculture has facilitated the emergence of zoonotic agents and the spread of non-native pests (Hulme 2020).
Unfortunately, whereas some aspects of public health ensuing from the introduction of human pathogens and vector mosquitoes are managed, others, including potential vertebrate hosts and ectoparasites, are less effectively addressed. Therefore, an integrated approach to biosecurity that addresses both species invasions and emerging infectious pathogens appears necessary. The research, stakeholder, and policymaker communities are rapidly beginning to understand the need for better integration between disciplines. This includes initiatives such as One Health, which has a goal to achieve optimal public health outcomes by monitoring and managing the interactions between humans, animals, and their environment. Likewise, the Planetary Health Alliance seeks to determine the human health consequences of human-caused disruptions of Earth's natural systems (Myers 2017). Nevertheless, neither One Health nor Planetary Health adequately captures the underlying nature of invasions by human pathogens and their relationship with invasive non-native species. A more robust framework can be provided by the concept of One Biosecurity, which, in addition to increasing the synergies between human health and invasion science, aims to refocus discussions toward practical tools and policies for preventing, eradicating, and containing biosecurity risks (Hulme 2020). The possibility of implementing the One Biosecurity concept has been further elaborated to highlight how international public health policy can be adapted to address much wider biosecurity risks stemming from invasive non-native pathogens, plants, and animals through developing new risk assessment tools that look beyond national borders toward biosecurity risks of international concern, a stronger regulatory instrument to address biosecurity threats at a worldwide scale, and the establishment of an international biosecurity convention responsible for biosecurity governance (Hulme 2021).
Management actions. Management actions against epidemics follow the same steps as in invasions: prevention, early detection, containment, control and eradication, and long-term management (Dunn and Hatcher 2015, Robertson et al. 2020). Many countries have in place early detection and rapid response systems, but the administrations in charge are usually not the same, with public health institutions to prevent epidemics, separated from environmental bodies to avert invasions. Successful management prospects decrease with the amount of time elapsed since the onset of the invasion or pathogen emergence (figure 4). Because of the rapid range expansion of many invasive species and pathogens, the window of opportunity for early detection and response is often very short. Control is usually the action that takes most of the time and effort. Eradication is difficult to achieve, except in small or remote areas and if actions start at early stages of invasion (Pluess et al. 2012). Prompt detection and control of emerging pathogens requires proper tracing of infected hosts independently of whether they are symptomatic or not. Eradication is very difficult when infected hosts are widespread and often requires vaccination of 50%–90% of the population, depending on how contagious the pathogen might be to achieve herd immunity. A major difference between an epidemic and an invasion is that when an epidemic takes place at a given locality, all of these management strategies might need to be set up simultaneously. That is, within a human population, different groups of people need to take different precautions or treatment measures, depending on their exposure to the pathogen. In a pandemic, all management practices need to be scaled up at once, both within and among populations of different regions. Conversely, because the rate of expansion of an invader follows a slower pace than that of a pathogen (figure 5), its management is more aligned with the stage of invasion than in epidemics.
Risk assessments. To inform managers and policymakers, research on biological invasions provides semiquantitative risk assessment tools to identify and prioritize species likely to become invasive and cause damage. Risk assessments also seek to identify the most susceptible habitats to invasion by a particular—or several—invasive species, through consideration of both species traits and recipient ecosystem characteristics. In human epidemics, the focus of the risk analysis is primarily on a particular pathogen, albeit multiple hosts and the risk of contagion and spread, is based on the traits of the pathogen and the demographic characteristics (e.g., gender, age, activity) of the receptive human host population. Spatially explicit risk assessments of invasion are very common and mainly rely on land-use and climate correlates between the native and the introduced area. These risk analyses have been implemented in vector-borne pathogens but could also be conducted for emerging pathogens albeit human population density and movement patterns seem to be better predictors of disease vulnerability than environmental characteristics (Jones et al. 2008). Models such as the EFBI that view ecosystems as hosts that differ in exposure, susceptibility, infectivity, and rates of recovery could potentially be a basis for parallel risks assessments for invasive species and human pathogens because they explicitly link the transmission of invasive species between ecosystems and rather than derive an arbitrary score or probability on invasion likelihood, risk assessment tools could be designed to estimate R0 (Hulme et al. 2020).
The evaluation of the impacts caused by epidemics focuses on the rates of infected people and fatalities, which are used to compare pathogens, regions, and management responses. However, as in invasions, which consequences extend beyond environmental impacts, the consequences of epidemics extend beyond health, both having socioeconomic impacts (Dobson et al. 2020). Attempts to quantify socioeconomic impacts in monetary terms are unlikely to provide a useful basis for evaluating and comparing the impacts of invasive species and pathogens, because they are extremely difficult to estimate and may neglect important aspects of human well-being. In invasions, there are many standardized impact assessment protocols that allow objective and transparent ways to rank and identify the worst invasive species. Notably, the socioeconomic impact classification of alien taxa (SEICAT; Bacher et al. 2017) classifies invasive species on the basis of the magnitude of their impacts on human well-being, on the basis of the capability approach from welfare economics (Robeyns 2011). In SEICAT, impacts are assigned to one of five levels—from minimal concern to massive—according to semiquantitative scenarios that describe the severity of the impacts on security, material and nonmaterial assets, health, freedom of choice and action, and social, spiritual, and cultural relations. All these impacts apply to any epidemic, and SEICAT could therefore be used to summarize and compare their impacts at national, regional or global scales.
Conclusions
In recent decades, we have witnessed how human activities that are poorly regulated can drive harmful invasive species and pathogen outbreaks (Perrings et al. 2002, Stein 2020). The epidemiology of human pathogens and invasion biology share many of the same mechanisms, phenomena, and challenges but also potential solutions (table 3). Global trade and travel are prime causes for the introduction of invasive species and pathogens, for invasive vertebrate reservoirs, and for invasive insect vectors. Even the patterns and dynamics of spread of reemerging “native” diseases, such as Ebola in West Africa and dengue in Southeast Asia, share similarities to those of invasive species. Many of the pathogens that cause these diseases can quickly become pandemics and then go through the same stages as invasive species. Much theory and many empirical insights gained in invasion biology can be extended to the study of emerging pathogens; similarly, invasion biology can immensely benefit from insights gained on the study of emerging human infectious pathogens. The amount and quality of the data collected on human infectious pathogens are undoubtedly much more refined than those available for other invasive species, as has been shown for SARS-CoV-2 (Bertelsmeier and Ollier 2020).
Comparison of main features and established concepts between biological invasions and human epidemics.
| Feature . | Biological invasions . | Human epidemics . | References . |
|---|---|---|---|
| Biogeographic and evolutionary origin | Non-native species from a region in which they could not be dispersed without human agency | Non-native pathogens dispersed directly or indirectly by humans or emerging native pathogens. Crossing a species barrier rather than a biogeographic barrier | Jones et al. 2008, Pyšek et al. 2017 |
| Routes of dispersal | Pathways | Routes of infection | Wolfe et al. 2007, Hulme et al. 2008, Saul et al. 2017 |
| Intentional: release and escape | Unintentional: vector borne, zoonotic, human contact, indirect contact by ingestion or the environment | ||
| Unintentional: contaminant, stowaway, corridor and unaided | Also intentional: historical cases during colonization of new territories, bioterrorism and anthrax mailing | ||
| Founder populations | Repeated introductions from several populations, genetically diverse (admixtures) | Few introductions from a single or few populations | |
| Stages | Transport, introduction, establishment, spread | Exposure, infection, transmission, epidemic spread; zoonotic spillover | Woolhouse and Gaunt 2007, Blackburn et al. 2011, Jeschke et al. 2013 |
| Spread rates and time lags | 0.1–102 km per year, years–decades | 103–104 km per year, days–decades | Kowarik 1995, McCallum et al. 2003 See figure 4 |
| Main studied causes of non-native species performance and impact | Traits of the organism (invasiveness), biotic and abiotic characteristics of the recipient ecosystem (invasibility) and the intensity and frequency of introduced individuals (propagule pressure) | Traits of the organism (pathogenicity), host age, genetics, physiology, immunity and people behavior | Lonsdale 1999, Mack et al. 2000, Enders et al. 2020 |
| Forecasting models’ focus and explanatory variables | On the invasive species. Environmental and proxies for propagule pressure as explanatory variables | On infected people (not the pathogen). Human demographics including movement and pathogen transmission as explanatory variables | See table 2 |
| Traditional impact focus | Biodiversity, environment, agriculture and farming | Medicine, public health | Jeschke et al. 2013, Vilà and Hulme 2017 |
| Traditionally involved management sectors | Environment, agriculture and farming, veterinary, water resources, trading | Public health, food, foreign affairs, traveling, veterinary, water resources | Ogden et al. 2019 |
| Feature . | Biological invasions . | Human epidemics . | References . |
|---|---|---|---|
| Biogeographic and evolutionary origin | Non-native species from a region in which they could not be dispersed without human agency | Non-native pathogens dispersed directly or indirectly by humans or emerging native pathogens. Crossing a species barrier rather than a biogeographic barrier | Jones et al. 2008, Pyšek et al. 2017 |
| Routes of dispersal | Pathways | Routes of infection | Wolfe et al. 2007, Hulme et al. 2008, Saul et al. 2017 |
| Intentional: release and escape | Unintentional: vector borne, zoonotic, human contact, indirect contact by ingestion or the environment | ||
| Unintentional: contaminant, stowaway, corridor and unaided | Also intentional: historical cases during colonization of new territories, bioterrorism and anthrax mailing | ||
| Founder populations | Repeated introductions from several populations, genetically diverse (admixtures) | Few introductions from a single or few populations | |
| Stages | Transport, introduction, establishment, spread | Exposure, infection, transmission, epidemic spread; zoonotic spillover | Woolhouse and Gaunt 2007, Blackburn et al. 2011, Jeschke et al. 2013 |
| Spread rates and time lags | 0.1–102 km per year, years–decades | 103–104 km per year, days–decades | Kowarik 1995, McCallum et al. 2003 See figure 4 |
| Main studied causes of non-native species performance and impact | Traits of the organism (invasiveness), biotic and abiotic characteristics of the recipient ecosystem (invasibility) and the intensity and frequency of introduced individuals (propagule pressure) | Traits of the organism (pathogenicity), host age, genetics, physiology, immunity and people behavior | Lonsdale 1999, Mack et al. 2000, Enders et al. 2020 |
| Forecasting models’ focus and explanatory variables | On the invasive species. Environmental and proxies for propagule pressure as explanatory variables | On infected people (not the pathogen). Human demographics including movement and pathogen transmission as explanatory variables | See table 2 |
| Traditional impact focus | Biodiversity, environment, agriculture and farming | Medicine, public health | Jeschke et al. 2013, Vilà and Hulme 2017 |
| Traditionally involved management sectors | Environment, agriculture and farming, veterinary, water resources, trading | Public health, food, foreign affairs, traveling, veterinary, water resources | Ogden et al. 2019 |
Comparison of main features and established concepts between biological invasions and human epidemics.
| Feature . | Biological invasions . | Human epidemics . | References . |
|---|---|---|---|
| Biogeographic and evolutionary origin | Non-native species from a region in which they could not be dispersed without human agency | Non-native pathogens dispersed directly or indirectly by humans or emerging native pathogens. Crossing a species barrier rather than a biogeographic barrier | Jones et al. 2008, Pyšek et al. 2017 |
| Routes of dispersal | Pathways | Routes of infection | Wolfe et al. 2007, Hulme et al. 2008, Saul et al. 2017 |
| Intentional: release and escape | Unintentional: vector borne, zoonotic, human contact, indirect contact by ingestion or the environment | ||
| Unintentional: contaminant, stowaway, corridor and unaided | Also intentional: historical cases during colonization of new territories, bioterrorism and anthrax mailing | ||
| Founder populations | Repeated introductions from several populations, genetically diverse (admixtures) | Few introductions from a single or few populations | |
| Stages | Transport, introduction, establishment, spread | Exposure, infection, transmission, epidemic spread; zoonotic spillover | Woolhouse and Gaunt 2007, Blackburn et al. 2011, Jeschke et al. 2013 |
| Spread rates and time lags | 0.1–102 km per year, years–decades | 103–104 km per year, days–decades | Kowarik 1995, McCallum et al. 2003 See figure 4 |
| Main studied causes of non-native species performance and impact | Traits of the organism (invasiveness), biotic and abiotic characteristics of the recipient ecosystem (invasibility) and the intensity and frequency of introduced individuals (propagule pressure) | Traits of the organism (pathogenicity), host age, genetics, physiology, immunity and people behavior | Lonsdale 1999, Mack et al. 2000, Enders et al. 2020 |
| Forecasting models’ focus and explanatory variables | On the invasive species. Environmental and proxies for propagule pressure as explanatory variables | On infected people (not the pathogen). Human demographics including movement and pathogen transmission as explanatory variables | See table 2 |
| Traditional impact focus | Biodiversity, environment, agriculture and farming | Medicine, public health | Jeschke et al. 2013, Vilà and Hulme 2017 |
| Traditionally involved management sectors | Environment, agriculture and farming, veterinary, water resources, trading | Public health, food, foreign affairs, traveling, veterinary, water resources | Ogden et al. 2019 |
| Feature . | Biological invasions . | Human epidemics . | References . |
|---|---|---|---|
| Biogeographic and evolutionary origin | Non-native species from a region in which they could not be dispersed without human agency | Non-native pathogens dispersed directly or indirectly by humans or emerging native pathogens. Crossing a species barrier rather than a biogeographic barrier | Jones et al. 2008, Pyšek et al. 2017 |
| Routes of dispersal | Pathways | Routes of infection | Wolfe et al. 2007, Hulme et al. 2008, Saul et al. 2017 |
| Intentional: release and escape | Unintentional: vector borne, zoonotic, human contact, indirect contact by ingestion or the environment | ||
| Unintentional: contaminant, stowaway, corridor and unaided | Also intentional: historical cases during colonization of new territories, bioterrorism and anthrax mailing | ||
| Founder populations | Repeated introductions from several populations, genetically diverse (admixtures) | Few introductions from a single or few populations | |
| Stages | Transport, introduction, establishment, spread | Exposure, infection, transmission, epidemic spread; zoonotic spillover | Woolhouse and Gaunt 2007, Blackburn et al. 2011, Jeschke et al. 2013 |
| Spread rates and time lags | 0.1–102 km per year, years–decades | 103–104 km per year, days–decades | Kowarik 1995, McCallum et al. 2003 See figure 4 |
| Main studied causes of non-native species performance and impact | Traits of the organism (invasiveness), biotic and abiotic characteristics of the recipient ecosystem (invasibility) and the intensity and frequency of introduced individuals (propagule pressure) | Traits of the organism (pathogenicity), host age, genetics, physiology, immunity and people behavior | Lonsdale 1999, Mack et al. 2000, Enders et al. 2020 |
| Forecasting models’ focus and explanatory variables | On the invasive species. Environmental and proxies for propagule pressure as explanatory variables | On infected people (not the pathogen). Human demographics including movement and pathogen transmission as explanatory variables | See table 2 |
| Traditional impact focus | Biodiversity, environment, agriculture and farming | Medicine, public health | Jeschke et al. 2013, Vilà and Hulme 2017 |
| Traditionally involved management sectors | Environment, agriculture and farming, veterinary, water resources, trading | Public health, food, foreign affairs, traveling, veterinary, water resources | Ogden et al. 2019 |
A cross-disciplinary perspective on infectious diseases and invasion biology could advance both fields. We advocate for an One Biosecurity (sensu Hulme 2020, 2021) approach to develop a unified framework for studying the pathways of introduction and the consequences of eco-evolutionary novelty, to compile and harmonize databases and information systems on major invasions and epidemics, to share predictive modeling skills of the spread and impacts of invasive species based not only on species traits but also on environmental characteristics, and to discuss institutional approaches and protocols in horizon scanning, risk assessments, systematic surveillance, and monitoring of invasions and epidemics.
Undoubtedly, globalization and the movement of organisms across biogeographic barriers are not only threatening biodiversity but also directly affecting human well-being through an array of new emerging infectious threats. Invasion biology has accumulated over recent decades many insights that could help improve the way we deal with these pathogens and the diseases they cause, but crossing this disciplinary bridge requires more tangible collaborations and concrete policy initiatives. Scientists, governments, and institutions should promote the cross-disciplinary approach to further advance in understanding the increasing threats of these novel entities and improve prevention and response measurements.
Acknowledgments
This review was inspired by CSIC PTI Salud Global. We gratefully acknowledge comments from Richard Mack and two anonymous reviewers to a previous version of the manuscript. This study was supported by the 2017–2018 Belmont Forum—BiodivERsA International joint call projects InvasiBES and AlienScenarios under the BiodivScen ERA-Net COFUND program and with the following funding organizations: the Spanish Ministry of Science and Innovation (grants no. PCI2018-092986 and no. PCI2018-092939, MCI/AEI/FEDER), the German Federal Ministry of Education and Research (grant no. 01LC1803A), and the Austrian Science Foundation (grant no. I 4011-B32). AP was funded by Chilean Comisión Nacional de Investigación Científica y Tecnológica grant no. PIA AFB170008, AMD by the UK Natural Environment Research Council grant no. NE/P016766/1, EGD by the Spanish Ministry of Science and Innovation grant no. PID2019-111109RB-I00 and Ramón y Cajal grant no. RYC-2013-13445, AR by NSERC grant no. RGPIN-2016-03918, and BG by Ramón y Cajal grant no. RYC2018-025160-I.
Montserrat Vilà (montse.vila@ebd.csic.es, ORCID: 0000-0003-3171-8261) is affiliated with the Estación Biológica de Doñana (EBD-CSIC) and with the Department of Plant Biology and Ecology, University of Sevilla, in Sevilla, Spain. Alison M. Dunn (ORCID: 0000-0002-4855-1077) is affiliated with the Faculty of Biological Sciences at the University of Leeds, in Leeds, in the United Kingdom. Franz Essl is affiliated with the Bioinvasions Global Change Macroecology Group, in the Department of Botany and Biodiversity Research at the University of Vienna, in Vienna, Austria. Elena Gómez-Díaz (ORCID: 0000-0002-4146-9003) is affiliated with the Institute of Parasitology and Biomedicine Lopez-Neyra, in Granada, Spain. Philip E. Hulme (ORCID: 0000-0001-5712-0474) is affiliated with the Bio-Protection Research Centre, at Lincoln University, in Canterbury, New Zealand. Jonathan M. Jeschke (ORCID 0000-0003-3328-4217) is affiliated with the Leibniz Institute of Freshwater Ecology and Inland Fisheries, with the Institute of Biology at Freie Universität Berlin, and with the Berlin-Brandenburg Institute of Advanced Biodiversity Research, in Berlin, Germany. Martín A. Núñez (ORCID: 0000-0003-0324-5479) is affiliated with the Grupo de Ecología de Invasiones, INIBIOMA, CONICET, at the Universidad Nacional del Comahue, in San Carlos de Bariloche, Argentina and with the Department of Biology and Biochemistry at the University of Houston, in Houston, Texas, in the United States. Richard S. Ostfeld (ORCID: 0000-0002-3707-9301 ORCID: 0000-0003-1284-3163) is affiliated with the Cary Institute of Ecosystem Studies, in Millbrook, New York, in the United States. Aníbal Pauchard is affiliated with the Laboratorio de Invasiones Biológicas in the Facultad de Ciencias Forestales, at the Universidad de Concepción, in Concepción, Chile, and with the Institute of Ecology and Biodiversity, in Santiago, Chile. Anthony Ricciardi (ORCID: 0000-0003-1492-0054) is affiliated with the Redpath Museum, at McGill University, in Montreal, Quebec, Canada. Belinda Gallardo (ORCID: 0000-0003-1492-0054) is affiliated with the Pyrenean Institute of Ecology, in Zaragoza, Spain, and with the BioRISC (Biosecurity Research Initiative at St Catharine's), at St Catharine's College, in Cambridge, in the United Kingdom.