-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Gargiulo, Andreas Goette, Jan Tijssen, Lars Eckardt, Thorsten Lewalter, Pascal Vranckx, Marco Valgimigli, Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials, European Heart Journal, Volume 40, Issue 46, 7 December 2019, Pages 3757–3767, https://doi.org/10.1093/eurheartj/ehz732
Close - Share Icon Share
Abstract
To investigate the safety and efficacy of double vs. triple antithrombotic therapy (DAT vs. TAT) in patients with atrial fibrillation (AF) and acute coronary syndrome or who underwent percutaneous coronary intervention (PCI).
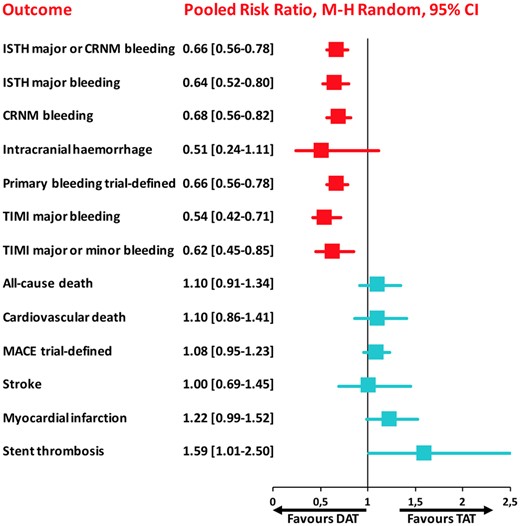
A systematic review and meta-analysis was performed using PubMed to search for non-vitamin K antagonist oral anticoagulant (NOAC)-based randomized clinical trials comparing DAT vs. TAT in AF patients undergoing PCI. Four trials encompassing 10 234 patients (DAT = 5496 vs. TAT = 4738) were included. The primary safety endpoint (ISTH major or clinically relevant non-major bleeding) was significantly lower with DAT compared with TAT [risk ratio (RR) 0.66, 95% confidence interval (CI) 0.56–0.78; P < 0.0001; I 2 = 69%], which was consistent across all available bleeding definitions. This benefit was counterbalanced by a significant increase of stent thrombosis (RR 1.59, 95% CI 1.01–2.50; P = 0.04; I 2 = 0%) and a trend towards higher risk of myocardial infarction with DAT. There were no significant differences in all-cause and cardiovascular death, stroke and major adverse cardiovascular events. The comparison of NOAC-based DAT vs. vitamin K antagonist (VKA)-TAT yielded consistent results and a significant reduction of intracranial haemorrhage (RR 0.33, 95% CI 0.17–0.65; P = 0.001; I 2 = 0%).
Double antithrombotic therapy, particularly if consisting of a NOAC instead of VKA and a P2Y12 inhibitor, is associated with a reduction of bleeding, including major and intracranial haemorrhages. This benefit is however counterbalanced by a higher risk of cardiac—mainly stent-related—but not cerebrovascular ischaemic occurrences.
Introduction
Most patients with atrial fibrillation (AF) and risk factors for stroke require oral anticoagulation (OAC) to prevent cerebrovascular or systemic embolism.1 Frequently AF coincides with coronary artery disease (CAD) and microcirculatory flow abnormalities,2 so many of these patients present with acute coronary syndrome (ACS) or stable CAD requiring percutaneous coronary intervention (PCI).1 , 3 The optimal antithrombotic treatment regimen for patients with AF undergoing PCI is a clinical conundrum. Dual antiplatelet therapy (DAPT) is recommended to reduce the risk of ischaemic complications in patients undergoing PCI and the combination of OAC with DAPT, a strategy generally called triple antithrombotic therapy (TAT), increases the bleeding risks compared with the use of OAC or DAPT alone.1 , 3–6 Therefore, research has focused on choosing a treatment strategy that provides the optimal balance between ischaemic and bleeding occurrences. Extensive evidence has shown that, compared with vitamin K antagonists (VKAs), non-VKA oral anticoagulants (NOACs), including dabigatran, rivaroxaban, apixaban, or edoxaban, decrease the risk of bleeding in patients with AF,3 , 7 and most recent trials have investigated the safety of double antithrombotic therapy (DAT) consisting of an OAC plus a P2Y12 inhibitor in comparison with TAT in the setting of patients with AF undergoing PCI or with ACS. In particular, Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention (PIONEER-AF PCI),8 Randomized Evaluation of Dual Antithrombotic Therapy With Dabigatran vs. Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI),9 Open-Label, 2 × 2 Factorial, Randomized, Controlled Clinical Trial to Evaluate the Safety of Apixaban vs. Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients with Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention (AUGUSTUS),10 and EdoxabaN TReatment versUS VKA in paTients with AF undergoing PCI (ENTRUST-AF PCI)11 trials have explored the role of NOACs in AF patients undergoing PCI or with ACS. However, none of these studies was powered to assess the effect of treatment on cerebrovascular or cardiac ischaemic events.
We conducted a meta-analysis of NOAC-based randomized trials comparing DAT vs. TAT in patients with AF and PCI or ACS.
Methods
Protocol
We followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting guidelines. We developed a protocol which was submitted to PROSPERO on 16 July 2019 and registered with the number CRD42019142779.
Search strategy and selection criteria
We performed a systematic search on PubMed from inception to 13 August 2019. The following keywords were used in different combinations: ‘percutaneous coronary intervention’, ‘PCI’, ‘coronary stenting’, ‘acute coronary syndrome’, ‘ACS’, ‘atrial fibrillation’, ‘AF’, ‘apixaban’, ‘rivaroxaban’, ‘edoxaban’, ‘dabigatran’, ‘vitamin k antagonist’, ‘warfarin’, ‘phenprocoumon’, ‘oral anticoagulation’, ‘oral anticoagulants’, ‘dual antithrombotic therapy’, ‘dual therapy’, ‘triple therapy’, ‘triple antithrombotic therapy’, ‘dual antiplatelet therapy’, ‘clopidogrel’ and ‘aspirin’, ‘randomised trial’. Relevant websites (i.e. clinicaltrialresult.org; clinicaltrials.gov; tctmd.com; cardiosource.com; theheart.org; escardio.org) and references of prior systematic reviews/meta-analyses were also screened for related studies. All randomized controlled trials in patients with AF who received PCI in at least 50% of the sample and were allocated to DAT consisting of any NOAC in combination with a P2Y12 inhibitor or TAT consisting of any VKA in combination with DAPT were included. Observational studies, registry data, ongoing trials without results, editorials, case series, and duplicate studies were excluded.
Citations were screened at the title and abstract level by two independent reviewers (G.G., M.V.), and full text was retrieved for those potentially eligible. The same authors extracted data of interest (from the published articles of PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS as well as their Supplementary material online; while data from ENTRUST-AF-PCI were internally available after database lock was achieved) into a structured dataset, and rated the risk of bias at the study level.8–11 All data were finally reviewed with other authors (A.G. and P.V.) and discrepancies, if any, were resolved by consensus.
Study outcomes
The primary safety bleeding endpoint was defined as International Society on Thrombosis and Haemostasis (ISTH) major bleeding or clinically relevant non-major bleeding (CRNMB) at longest available follow-up (between 6 and 14 months). Secondary safety outcomes included bleeding endpoints according to various definitions (trial-defined primary safety bleeding endpoint; ISTH major bleeding, ISTH CRNMB, thrombolysis in myocardial infarction (TIMI) major bleeding, TIMI major or minor bleeding, intracranial haemorrhage).
Secondary efficacy endpoints included all-cause death; cardiovascular death; trial-defined major adverse cardiovascular event (MACE); myocardial infarction (MI); stroke; and stent thrombosis (ST). Main endpoint definitions are displayed in Supplementary material online, Table S1.
Data synthesis and analysis
Effect sizes were calculated with the Mantel–Haenszel random-effects estimator and expressed as risk ratios (RRs) and 95% confidence intervals (CIs). Number needed to treat for benefit (NNTB) or harm (NNTH) were also calculated according to Cochrane’s recommendations: [1/ACRx(1−RR)] where ACR is the assumed control risk. Heterogeneity was assessed by I 2 tests, with substantial heterogeneity defined as I 2 greater than 50%. Sensitivity analyses were conducted to investigate the robustness of our results to assess whether any of the included studies had a large influence on the results. Given the peculiar design of the AUGUSTUS trial, we also conducted a secondary analysis to compare NOAC-based DAT vs. VKA-based TAT from the included trials (by excluding the VKA-based DAT and NOAC-based TAT arms from the AUGUSTUS). Analyses were also performed with a fixed-effect model to confirm results. An additional analysis was conducted to analyse separately the doses of dabigatran 110 mg and 150 mg b.i.d. for the RE-DUAL PCI trial.
The methodological quality of the randomized trials was assessed by Cochrane’s Collaboration tool for assessing risk of bias. For each trial, bias was assessed qualitatively as low, unclear, or high risk of bias by independent investigators. Due to the small number of studies (<10) included in the analysis, publication bias was not assessed. Statistical significance was set at P < 0.05 (two-tailed). Data were analysed by using Reviewer Manager (RevMan, version 5.3; Cochrane).
Results
Search results and study details
A total of 249 studies were screened for eligibility, out of which four randomized trials8–11 including 10 234 patients (DAT = 5496 vs. TAT = 4738) in AF patients following PCI were included in the final analysis (Supplementary material online, Figure S1).
The What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST)12 trial was excluded because only VKA was investigated and it included 31% of the patients without AF. We also excluded the Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation (ISAR-TRIPLE)13 trial because only VKA was investigated and no DAT regimen was implemented. Finally, the TAT arm of PIONEER AF-PCI8 composed of aspirin plus P2Y12 inhibitor plus low-dose rivaroxaban (n = 709 patients) was excluded because rivaroxaban 2.5 mg b.i.d. is not an approved regimen for thromboembolic protection in patients with AF.
Since the RE-DUAL PCI9 included two dabigatran-based DAT arms, at either 110 mg b.i.d or 150 mg b.i.d., the two dabigatran arms were pooled including a total of 1744 patients for the main analysis.
The characteristics of the four included trials are reported in Supplementary material online, Table S1, whilst patient characteristics by treatment arm are reported in Supplementary material online, Table S2.
All trials enrolled AF patients undergoing PCI, but AUGUSTUS,10 which included also AF patients with medically managed ACS (23.9%). Yet, the AUGUSTUS was the only study based on a factorial 2 × 2 design whereby aspirin (TAT) or placebo (DAT) was also randomly allocated in both OAC groups.
Across the four studies, DAT regimen consistently started at the time of randomization, which occurred from immediately after PCI up until 14 days after PCI and aspirin was used as standard of care at the time of intervention (Supplementary material online, Table S1). Clopidogrel was the most frequently used P2Y12 inhibitor (overall 90.1%). The duration of follow-up ranged from 6 to 14 months.
Study quality was high across all four studies (Supplementary material online, Table S3).
Safety endpoints
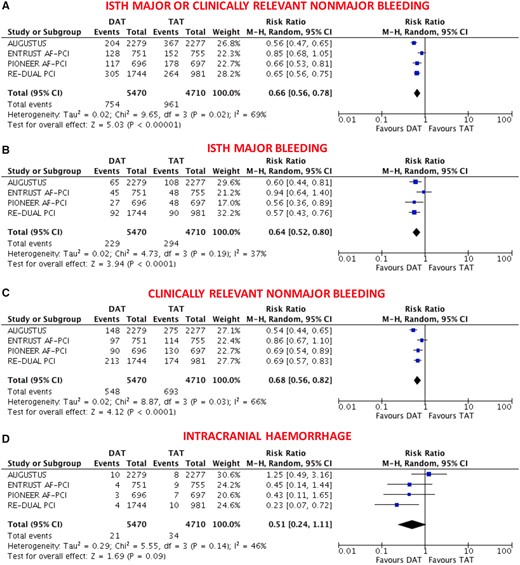
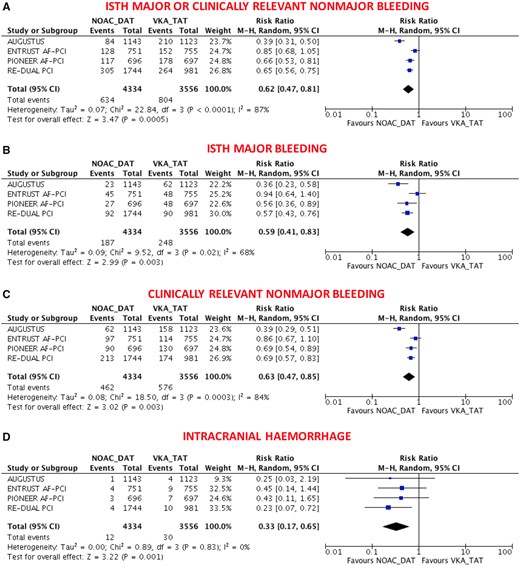
The primary safety bleeding endpoint of ISTH major bleeding or CRNMB was significantly reduced with DAT compared with TAT (13.4% vs. 20.8%; RR 0.66, 95% CI 0.56–0.78; P < 0.0001; I 2 = 69%; Figure 1A). This benefit was driven by reduction of both major (4.1% vs. 6.4%; RR 0.64, 95% CI 0.52–0.80; P < 0.0001; I 2 = 37%; Figure 1B) and CRNMB (9.8% vs. 14.9%; RR 0.68, 95% CI 0.56–0.82; P < 0.0001; I 2 = 66%; Figure 1C). The results remained consistent when the analysis was restricted to NOAC-based DAT vs. VKA-based TAT (Figure 2A–C) or when different bleeding definitions were implemented (Supplementary material online, Figures S2 and S3). DAT was associated with a non-significant 49% reduction of intracranial haemorrhage compared with TAT (0.40% vs. 0.74%; RR 0.51, 95% CI 0.24–1.11; P = 0.09; I 2 = 46%; Figure 1D), and this benefit became greater (67% reduction) and statistically significant when the analysis was restricted to NOAC-based DAT vs. VKA-based TAT (0.28% vs. 0.86%; RR 0.33, 95% CI 0.17–0.65; P = 0.001; I 2 = 0%; Figure 2D).
Main bleeding endpoints in double vs. triple antithrombotic therapy. (A) ISTH Major or clinically relevant non-major bleeding; (B) ISTH major bleeding; (C) clinically relevant non-major bleeding; (D) intracranial haemorrhage. Random-effects risk ratios and 95% confidence intervals for main bleeding endpoints. CRNMB, clinically relevant non-major bleeding; DAT, double antithrombotic therapy; ISTH, International Society on Thrombosis and Haemostasis; M–H, Mantel–Haenszel; PCI, percutaneous coronary intervention; TAT, triple antithrombotic therapy. Note: PIONEER AF-PCI provided individual endpoints of ISTH major and CRNMB but not the composite that was derived by the sum of these two. Re-DUAL PCI did not provide CRNMB that was derived by subtracting ISTH major by the composite.
Main bleeding endpoints in non-vitamin K antagonist oral anticoagulant-based double antithrombotic therapy vs. vitamin K antagonist-based triple antithrombotic therapy. (A) ISTH Major or clinically relevant non-major bleeding; (B) ISTH major bleeding; (C) clinically relevant non-major bleeding; (D) intracranial haemorrhage. Random-effects risk ratios and 95% confidence intervals for main bleeding endpoints. DAT, double antithrombotic therapy; ISTH, International Society on Thrombosis and Haemostasis; M–H, Mantel–Haenszel; NOAC, non-vitamin K antagonist oral anticoagulant; PCI, percutaneous coronary intervention; TAT, triple antithrombotic therapy; VKA, vitamin K antagonist.
Efficacy endpoints
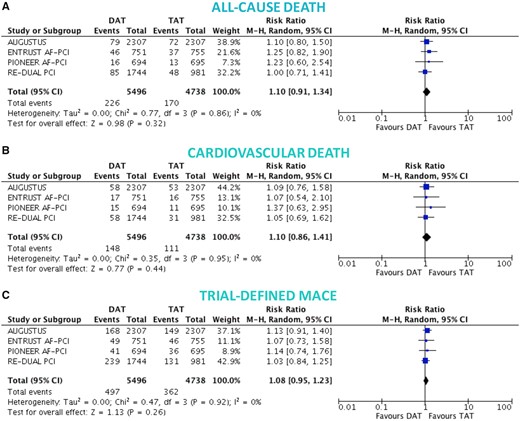
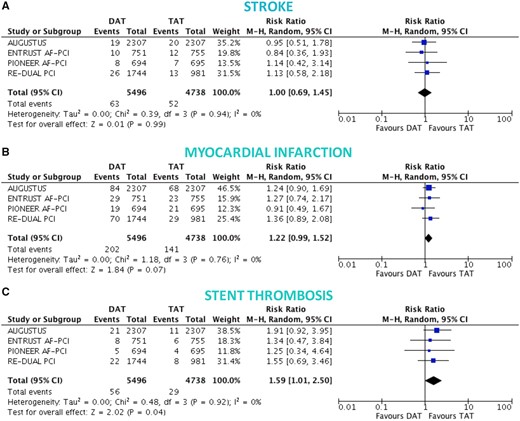
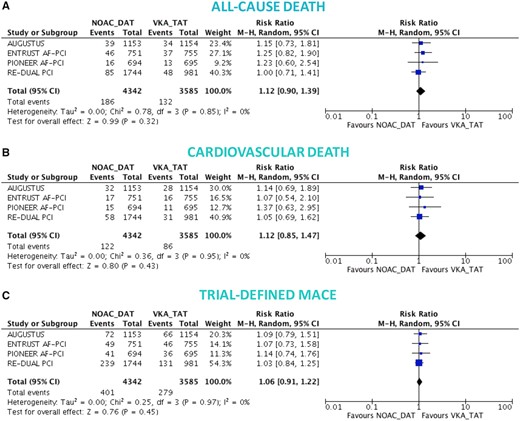
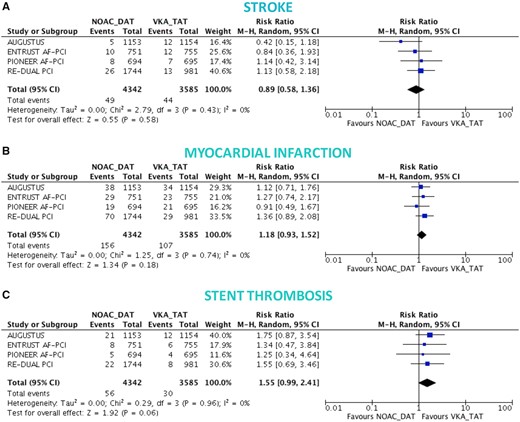
There was no significant difference between DAT and TAT in terms of all-cause death (4.0% vs. 3.7%; RR 1.10, 95% CI 0.91–1.34; P = 0.32; I 2 = 0%; Figure 3A), cardiovascular death (2.6% vs. 2.4%; RR 1.10, 95% CI 0.86–1.41; P = 0.44; I 2 = 0%; Figure 3B), MACE (8.6% vs. 8.0%; RR 1.08, 95% CI 0.95–1.23; P = 0.26; I 2 = 0%; Figure 3C), and stroke (1.1% vs. 1.1%; RR 1.00, 95% CI 0.69–1.45; P = 0.99; I 2 = 0%; Figure 4A), while DAT was associated with a borderline higher risk of MI (3.6% vs. 3.0%; RR 1.22, 95% CI 0.99–1.52; P = 0.07; I 2 = 0%; Figure 4B), and a nominally significantly higher risk of ST (1.0% vs. 0.6%; RR 1.59, 95% CI 1.01–2.50; P = 0.04; I 2 = 0%; Figure 4C). These results were consistent when the analysis was restricted to NOAC-based DAT vs. VKA-based TAT (Figures 5 and 6).
Death and major adverse cardiovascular events in double vs. triple antithrombotic therapy. Random-effects risk ratios and 95% confidence intervals for all-cause death (A), cardiovascular death (B), and major adverse cardiovascular events (C). Abbreviations as Figure 1.
Ischaemic endpoints in double vs. triple antithrombotic therapy. Random-effects risk ratios and 95% confidence intervals for stroke (A), myocardial infarction (B), and ST (C). Note: stent thrombosis definitions were not overlapping, and numbers refer to definite ST for the RE-DUAL PCI and ENTRUST AF-PCI trials, likely definite ST also for PIONEER-AF PCI (not clearly reported, but low numbers suggest this), and definite or probable ST for AUGUSTUS. Abbreviations as Figure 1.
Death and major adverse cardiovascular events in non-vitamin K antagonist oral anticoagulant-based double antithrombotic therapy vs. vitamin K antagonist-based triple antithrombotic therapy. Random-effects risk ratios and 95% confidence intervals for all-cause death (A), cardiovascular death (B), and major adverse cardiovascular events (C). Abbreviations as Figure 1.
Ischaemic endpoints in non-vitamin K antagonist oral anticoagulant-based double antithrombotic therapy vs. vitamin K antagonist-based triple antithrombotic therapy. Random-effects risk ratios and 95% confidence intervals for stroke (A), myocardial infarction (B), and stent thrombosis (C). Note: number of events of stent thrombosis stratified by NOAC-DAT vs. VKA-TAT for the AUGUSTUS come from a recent meta-analysis and likely correspond to definite or probable or possible ST.
Additional analyses
Bleeding endpoints remained consistent for both comparisons of DAT vs. TAT and NOAC-based DAT vs. VKA-based TAT when dabigatran 110 mg or 150 mg were analysed separately (Supplementary material online, Figures S4–S9). There was a slightly greater signal of higher cardiac ischaemic risk in the DAT or NOAC-based DAT groups, mainly in terms of higher MI or ST, when dabigatran 110 mg, but not dabigatran 150 mg, was included in the analysis (Supplementary material online, Figures S10–S15).
The primary bleeding endpoint of this meta-analysis remained consistent removing one study at a time (Supplementary material online, Table S4).
Overall results remained entirely consistent when pooled RRs were estimated though a fixed-effect model (Supplementary material online, Table S5).
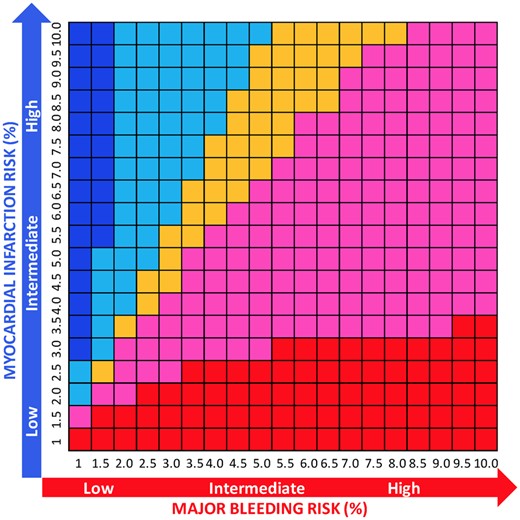
The NNTB and NNTH were calculated for safety and efficacy endpoints, respectively (Supplementary material online, Table S6). We also calculated NNTB and NNTH for multiple risk strata for both ISTH major bleeding and MI and analysed the net benefit (NNTB < NNTH) or harm (NNTB > NNTH) (Figure 7; Supplementary material online, Table S7).
Number needed to treat for benefit or harm for double vs. triple antithrombotic therapy according to risk of major bleeding and myocardial infarction. At each risk (%) ranging from 1% to 10% for both major bleeding and myocardial infarction, the difference between NNTB and NNTH was calculated. Red indicates that the net benefit of double vs. triple antithrombotic therapy is in favour of bleeding (NNTB < NNTH, thus reduction of bleeding is higher than the risk of myocardial infarction) and its intensity refers to greater (dark red) or lower (light red/pink) benefit (with the cut-off range selected arbitrarily to be −100 to 100), while blue indicates a net ischaemic harm (NNTB > NNTH, thus the reduction of bleeding is lower than the risk of myocardial infarction) and its intensity refers to greater (dark blue) or lower (light blue) harm (with the cut-off range selected arbitrarily to be −100 to 100). Orange indicates a neutral effect (NNTB = NNTH; we arbitrarily selected a range from −10 to 10 to consider the effect as neutral).
Discussion
The present systematic review and meta-analysis of all of the so far planned and executed large international NOAC studies examined the role of DAT vs. TAT in 10 234 patients AF patients undergoing PCI or with ACS. Our main findings can be summarized as follows:
Double antithrombotic therapy, compared with TAT, significantly reduced major and CRNMB, but only NOAC-based DAT was associated with a significant decrease of intracranial haemorrhage.
Double antithrombotic therapy was associated with similar rates of trial-defined MACE, all-cause or cardiovascular death and stroke when compared with TAT; however, it increased the risk of myocardial infarction and ST, with a statistical borderline and significant effect, respectively. These risks appeared slightly greater when dabigatran 110 mg, but not dabigatran 150 mg, was separately appraised in the DAT group.
The WOEST study was the first to assess the effect of aspirin removal after intervention on bleeding endpoints as compared to standard VKA-based TAT.12 This pivotal trial included 573 patients and showed that the combination of clopidogrel and VKA was associated with a significant reduction of any bleeding without an apparent increase, and actually with a numerical decrease, of thrombotic events such as MI or ST when compared with standard TAT. A nominally significant reduction of overall mortality was similarly observed in favour of DAT. However, this study included 31% of patients with indications to OAC other than AF (i.e. mechanical valves, apical aneurysm, etc.), it did not include NOAC-based therapy, and 67% of patients received TAT for 1-year in the control arm, which is a TAT duration no longer supported by guidelines.1 , 3 , 4 Then, ISAR-TRIPLE was performed, but it did not include patients treated with NOACs and did not compare a DAT vs. TAT regimen but rather two different TAT regimens.
Four NOACs have been tested in head-to-head studies against VKA in AF patients and a pooled analysis showed lower bleeding risks, including intracranial but not gastrointestinal occurrences, as well as thromboembolic and mortality risks associated with the former as compared to the latter.7
All four NOACs were then investigated in context of WOEST-like studies, where each of them was tested in combination with a P2Y12 inhibitor, largely consisting of clopidogrel, against VKA-based TAT, with the only exception of the AUGUSUTUS study.8–11
When separately appraised, each of these four studies showed evidence for lower bleeding risks in the DAT group, without apparent trade-off for ischaemic events. However, ischaemic events, both cerebrovascular or cardiac, are roughly 10-fold less prevalent in contemporary practice as compared to the bleeding occurrences which were captured in the primary endpoint of these studies.8–11 As a result, each of these individual studies is largely underpowered to detect a potentially clinically meaningful difference in cardiac or cerebrovascular ischaemic events between treatment strategies.
Even in a prior meta-analysis, which included only two of the four available NOAC studies, the upper boundary of the 95% CI entails a two-fold higher risks of MI with DAT when compared with TAT.14 , 15 Our meta-analysis, at least partially, overcomes this issue; it includes all of the so far planned and executed multinational NOAC AF/PCI or ACS trials and is based on roughly twice as many events as compared to the previous one, particularly for individual ischaemic endpoints such as myocardial infarction and ST or the key safety endpoint of intracranial bleeding.
Our current analysis confirms previous findings on the advantage of DAT over TAT with respect to bleeding complications.8–12 , 14 , 16 , 17 However, this is the first pooled analysis to show a significant decrease of intracranial haemorrhage with DAT as compared to TAT.14 , 16 , 17 This finding is consistent with prior NOAC studies focusing on AF but non-PCI patients.7 This effect seems indeed to mainly (if not exclusively) come from the comparison of NOAC vs. VKA, as suggested by the fact that it reaches statistical significance only when the VKA-based DAT and the NOAC-based TAT in AUGUSTUS study is excluded from the analysis. Interestingly, there was high heterogeneity across trials with respect to the primary safety bleeding endpoint, which largely came from non-major bleeding events. Indeed, no heterogeneity was observed across trials with respect to intracranial bleeding when the VKA-based DAT and the NOAC-based TAT in AUGUSTUS study is excluded from the analysis.
A second novel and similarly clinically relevant finding from our meta-analysis is the observation that the bleeding benefit associated with DAT comes with a trade-off of cardiac, but not cerebrovascular, ischaemic events (Take home figure and Supplementary material online, Figure S16). This signal was missed in previous meta-analyses, which apparently supported the conclusion that DAT was not only safer but also as effective as TAT with respect to cardiac ischaemic endpoints.14 , 16 , 17 This finding carries relevant clinical and pathophysiological implications and reinforces the notion that the upfront selection between TAT or DAT and/or the optimal timing for aspirin discontinuation after invention or ACS should be individualized. The analysis of NNTB and NNTH based on individual risks of major bleeding and MI may help identifying the net benefit/harm for each individual patient (Figure 7). This analysis, however, highlights the concept that individual risks of bleeding and MI influence the overall risk/benefit ratio of each therapeutic strategy and should be taken into account, rather than providing a specific decision tool to select between DAT or TAT.
The summary of safety and efficacy endpoints in double vs. triple antithrombotic therapy demonstrating that double antithrombotic therapy is associated with reduction of bleeding events but with a trade-off of cardiac ischaemic complications. Pooled random-effects risk ratios with 95% confidence intervals for safety and efficacy endpoints. CRNM, clinically relevant non-major; DAT, double antithrombotic therapy; ISTH, International Society on Thrombosis and Haemostasis; MACE, major adverse cardiovascular events; TAT, triple antithrombotic therapy; TIMI, thrombolysis in myocardial infarction.
The mechanisms through which early aspirin discontinuation exposes patients to higher ischaemic risks remain speculative. From one side, it emphasizes the importance of COX-1 inhibition in the prevention of cardiovascular ischaemic events.18 , 19 On the other hand, dropping aspirin may expose clopidogrel poor- or non-metabolizers to insufficient P2Y12 inhibition early after index PCI or index ACS.20 Whether the use of ticagrelor or prasugrel in the context of DAT may minimize the ischaemic risks while preserving the bleeding benefit as compared to TAT remains to be studied.
When both dabigatran doses were separately appraised, the MI or ST risks observed with DAT were apparently slightly increased with the low (110 mg b.i.d.), but not the high (150 mg b.i.d.) dabigatran dose. This observation might suggest a lower protective effect of low-dose dabigatran on spontaneous or stent-related coronary thromboembolic occurrences, which is consistent with the results of the RE-LY trial.21 Alternatively, it may reflect a chance finding related to the low number of cardiovascular events in each dabigatran arm, when separately appraised.
Study limitations
This is an aggregate data meta-analysis, but individual patient data are not publicly available at the time, therefore, subgroup analyses exploring specific subsets of patients or the role of different variables across the trials is highly desirable. Stent thrombosis definition implemented was not fully uniform across trials. Specifically, definite ST was not available from the AUGUSTUS (definite/probable ST was available for the main comparison of DAT vs. TAT, while for the comparison of NOAC-based DAT vs. VKA-based TAT data were extracted by a recent meta-analysis which seems to include definite/probable/possible ST). Additionally, being a study-level meta-analysis, we do not have data on timing of ST occurrence. Finally, we could not run a secondary analysis on PCI-only patients because full outcome data from the AUGUSTUS in this population (by excluding 23.9% of medically-managed patients with ACS) are not yet available; however, we do not believe this might affect the concerns raised by the increased ST, rather the dilution due to ACS patients should reinforce the caution towards this DAT-related higher risk.
Conclusion
Compared with TAT, DAT, particularly when based on NOACs, is associated with a reduction in bleeding complications, including major and intracranial haemorrhages. However, this benefit is counterbalanced by a higher risk of ischaemic, mainly stent-related, events.
Funding
The ENTRUST-AF PCI study was sponsored and funded by Daiichi Sankyo Pharma Development and Daiichi Sankyo Europe. The present study did not receive any direct or indirect funding.
Conflict of interest: A.G. discloses honoraria and speaker fees from Astra Zeneca, Bayer Health Care, Berlin-Chemie, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Medtronic, Novartis, and Omeicos. Research has been supported by Josef-Freitag Stiftung and Deutsche Herzstiftung e.V. outside the submitted work. J.T. reports personal fees from AstraZeneca, Bayer, and Boehringer Ingelheim outside the submitted work. L.E. discloses consultant fees, speaking honoraria, and travel expenses from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boehringer, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Sanofi Aventis. Research has been supported by German Research Foundation (DFG) and German Heart Foundation outside the submitted work. T.L. reports personal fees from Abbott, Boston Scientific, Bayer, Boehringer, Daiichi Sankyo, and Pfizer outside the submitted work. P.V. discloses personal fees from Daiichi Sankyo, AstraZeneca, Bayer Health Care, and Terumo outside the submitted work. M.V. reports grants and personal fees from Abbott, Alvimedica, Amgen, Bayer, Bristol-Myers Squibb SA, Coreflow, Daiichi Sankyo, Vifor, Idorsia, Terumo, and iVascular; grants and personal fees from grants from AstraZeneca and Medicure, outside the submitted work. G.G. has nothing to disclose.
See page 3768 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz823)
References
Antithrombotic Trialists’ (ATT) Collaboration,
Author notes
Giuseppe Gargiulo and Andreas Goette authors contributed equally as first authors.
Pascal Vranckx and Marco Valgimigli authors contributed equally as senior authors.
- anticoagulants
- atrial fibrillation
- myocardial infarction
- fibrinolytic agents
- percutaneous coronary intervention
- ischemia
- intracranial hemorrhages
- hemorrhage
- cerebrovascular accident
- ischemic stroke
- safety
- vitamins
- heart
- antagonists
- cardiovascular event
- vitamin k antagonists
- stent thrombosis
- cardiovascular death
- surrogate endpoints
- p2y12 receptor antagonists
- international society of thrombosis and haemostasis
- direct oral anticoagulants