Abstract
Body-brain interaction provides a novel approach to understand neurodevelopmental conditions such as autism spectrum disorder (ASD). In this systematic review, we analyse the empirical evidence regarding coexisting differences in autonomic (ANS) and central nervous system (CNS) responses to social stimuli between individuals with ASD and typically developing individuals. Moreover, we review evidence of deviations in body-brain interaction during processing of socially relevant information in ASD. We conducted systematic literature searches in PubMed, Medline, PsychInfo, PsychArticles, and Cinahl databases (until 12.1.2022). Studies were included if individuals with ASD were compared with typically developing individuals, study design included processing of social information, and ANS and CNS activity were measured simultaneously. Out of 1892 studies identified based on the titles and abstracts, only six fulfilled the eligibility criteria to be included in synthesis. The quality of these studies was assessed using a quality assessment checklist. The results indicated that individuals with ASD demonstrate atypicalities in ANS and CNS signalling which, however, are context dependent. There were also indications for altered contribution of ANS-CNS interaction in processing of social information in ASD. However, the findings must be considered in the context of several limitations, such as small sample sizes and high variability in (neuro)physiological measures. Indeed, the methodological choices varied considerably, calling for a need for unified guidelines to improve the interpretability of results. We summarize the current experimentally supported understanding of the role of socially relevant body-brain interaction in ASD. Furthermore, we propose developments for future studies to improve incremental knowledge building across studies of ANS-CNS interaction involving individuals with ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body-brain interaction provides a novel approach to understand neuropsychiatric and neurodevelopmental conditions, such as autism spectrum disorder (ASD), more comprehensively. ASD is a neurodevelopmental condition characterised by deficits in social communication and interaction along with a variety of restricted repetitive behaviours, focused interests, and activities (American Psychiatric Association, 2013). Although there is a vast amount of literature regarding autonomic and central nervous system (ANS and CNS, respectively) atypicalities among individuals with ASD (e.g., Arora et al., 2021; Hutt et al., 1975; O’Reilly et al., 2017; Palkovitz & Wiesenfeld, 1980; Pardo & Eberhart, 2007), less is known about the interaction between ANS and CNS and their contribution to social information processing difficulties. Thus, investigating body-brain interaction in the context of social information processing could give a more elaborated understanding of the characteristics related to social functioning among individuals with ASD.
The anatomy and function of ANS have been studied for over 100 years (e.g., Langley, 1903). ANS is known to dynamically regulate the energy balance in body systems to adapt to environmental demands, that is, to maintain homeostasis, through the activation of the parasympathetic and sympathetic nervous systems (PSNS and SNS, respectively). While PSNS is predominant during conditions of rest promoting a state of recovery and energy conservation, activation of SNS prepares the organism to respond to challenging conditions and stressors resulting in a state of elevated activity or ‘fight or flight’ response. For historical reasons, these anatomically and functionally distinct branches of ANS are typically understood as a dichotomic system working in opposite directions. Although PSNS and SNS are indeed complementary in nature, their interaction is rather characterised by coactivity and reciprocity (Berntson & Cacioppo, 2004). The two branches of ANS and their dynamic interplay can be studied using recordings of pupil size, heart rate variability (HRV), skin conductance responses (SCR), and electrodermal activity (EDA) (Acharya et al., 2006; Berntson & Cacioppo, 2004; Mathôt, 2018; Posada-Quintero & Chon, 2020), that reflect the activation of PSNS and SNS to different degrees.
Although the role of ANS in homeostatic regulation is undisputable, its relevance for perception and processing of information is less established. A growing body of evidence suggests that ANS pathways interact with CNS even beyond homeostatic regulation, influencing affective and cognitive processes (Lacey & Lacey, 1978; Parviainen et al., 2022; Tsakiris & Critchley, 2016). For long, ANS activation has been considered to play a critical role in the experience of emotions (Damasio, 1998; Kreibig, 2010) and processing of emotional information (Quintana et al., 2012). During the past 10 years, the role of ANS in the perception and processing of non-emotional sensory information has been recognised and studied more comprehensively. A review by Critchley and Garfinkel (2018) addressed the manifold, yet tentative, interactions between physiology (e.g., cardiac, respiratory, and gastric activity) and cognitive processes (e.g., attention, perception, learning, and decision-making), and recent empirical studies have highlighted the coupling between the phase of bodily rhythms (e.g., cardiac cycle and respiration) and different perceptual and cognitive functions, such as oculomotor behaviour during visual sampling (Galvez-Pol et al., 2020) and encoding of information (Waselius et al., 2019). Since social functioning requires fluent processing of both emotional and non-emotional information, it is highly relevant to understand the role of ANS in processing of social information in more detail.
ANS pathways target several cortical areas (e.g., medial prefrontal cortex, PFC; anterior cingulate cortex, ACC; anterior insular cortex, AIC), some of which also seem to be relevant for processing of socially relevant information (Beissner et al., 2013; Frith & Frith, 2007; Van Overwalle, 2009). More specifically, the visceral target areas in the brain are shown to be linked with attaching value to objects, mentalizing, perspective taking and experience of emotions (Berntson & Khalsa, 2021; Frith & Frith, 2007; Van Overwalle, 2009). This gives a reason to consider the potential role of ANS-CNS interaction in processing of social information.
In typically developing individuals, emerging evidence suggests an interaction between neural and bodily rhythms. For instance, Herrero et al. (2018) have demonstrated links between the frequency of respiration and brain oscillatory activation, and Richter et al. (2017) have reported coupling between the phase of slow-oscillating gastric electric signalling and amplitude of the brain alpha oscillations. Moreover, Kluger and Gross (2020) have observed that the depth and phase of respiration modulate neural oscillatory activity. Besides the accumulating findings showing coupling between autonomic, enteric (governing gastrointestinal tract) and central nervous systems, the evidence from recent studies integrates the coupling between body and the brain with processing of external input (Kluger et al., 2021; Waselius et al., 2018), including processing of socially relevant information. For instance, Garfinkel et al. (2014) have shown that the phase of the cardiac cycle (i.e., diastole vs. systole) influences the neural processing of fear and threat. In addition, Candia-Rivera et al. (2022) have demonstrated that emotional stimuli modulate cardiac activity, which further plays a causal role in initiating a cortical response that is linked to emotions. Moreover, D’Hondt et al. (2010) have reported that early cortical responses to emotional pictures are significantly correlated with SCR. Eisenbarth et al. (2016), in turn, have found both common and measure-specific neural activation patterns predicting physiological responses to social threat. Aforementioned studies reflect the diversity of methodological approaches previously used to investigate body-brain interaction, and thus, an inclusive definition of body-brain interaction is emphasised in this systematic review as well.
It is generally assumed that deficits in social interaction and communication, the core features of ASD, may be due to atypical functional characteristics of the CNS. Indeed, accumulating body of evidence suggests that anatomical and functional neurobiological atypicalities are highly pervasive in ASD (Amaral et al., 2008; O’Reilly et al., 2017; Pardo & Eberhart, 2007; Stanfield et al., 2008; Wang et al., 2013). Recent meta-analyses have shown functional differences, such as hypoactivation, in brain regions relevant for social cognition (Di Martino et al., 2009; Patriquin et al., 2016) and in neural characteristics between individuals with ASD and typically developing individuals (Kang et al., 2018; Port et al., 2015). Especially, atypicalities regarding neural processing of social information, such as faces (e.g., Ammons et al., 2021; McPartland et al., 2004), emotions (e.g., Hall et al., 2003; Leung et al., 2018; Tseng et al., 2016), empathy (e.g., Fan et al., 2014; Schulte-Rüther et al., 2011) and joint attention (e.g., Redcay et al., 2013) have been observed among individuals with ASD. However, both similar brain activation between ASD and comparison groups as well as opposing findings have been reported, and thus, current evidence is considered ambiguous and inconsistent (e.g., Dufour et al., 2013; Hadjikhani et al., 2004; Pierce et al., 2004).
Interestingly, individuals with ASD have also been reported to demonstrate differential functional properties of ANS, such as chronic autonomic hypoarousal and hyperarousal (Bujnakova et al., 2016; Patriquin et al., 2019). According to a systematic review by Lydon et al. (2014), autonomic arousal during processing of socially relevant stimuli is also different among individuals with ASD when compared with typically developing individuals. For instance, lower amplitude of respiratory sinus arrhythmia (RSA), that is, the universally observed synchrony between respiration and heart rate, has been associated with increased experience of social stress (Cheng et al., 2020) and deficiencies in emotion recognition among individuals with ASD (Bal et al., 2010). Moreover, being an object of direct eye gaze has been shown to elicit differential patterns of arousal indexed by SCR in children with autism and in typically developing children (Kylliäinen & Hietanen, 2006). On the contrary, some studies have found no evidence of atypical autonomic responses to social stimuli in ASD (e.g., Louwerse et al., 2014). Generally, although some evidence exists, findings regarding ANS reactivity to social stimuli have been inconsistent and the relevance of ANS in social information processing remains unclear.
Taken together, there is extensive accumulating empirical evidence regarding neurobiological and functional atypicalities in the ANS and CNS among individuals with ASD. For approaching the relevance of these findings for behavioural features and experiences of individuals with ASD, it would be crucial to understand the association between ANS and CNS atypicalities. However, the studies focusing on both ANS and CNS functions in the same study, and especially their interaction, are more scattered. Indeed, research combining ANS and CNS measures in ASD does exist, but there is a lack of systematic examination of the accumulated evidence from the perspective of theoretically coherent rationale. Thus, there is a need for integrating the existing evidence of the interaction between ANS and CNS that may underlie social information processing difficulties among individuals with ASD.
Based on the existing theories and findings related to body-brain interaction in typically developing individuals, as well as literature regarding atypical neurophysiological reactivity and social information processing in individuals with ASD, we hypothesize that the characteristics of persons diagnosed with ASD may reflect altered ANS and CNS coupling during processing of sensory input, especially processing of socially relevant information. This assumption builds on the suggested role of ANS signalling in modulating initial perception of incoming stimuli and contributing to the cognitive top-down reappraisal of information flow also during social interaction (Badcock et al., 2017; Craig, 2014; Critchley & Harrison, 2013). Since ANS is evolutionarily older and automatically adjusts to internally or externally driven requirements (McEwen, 1998), our underlying assumption was that ANS activation modulates the processing of social information in the CNS. In other words, ANS could be regarded as a ‘filter’ that influences the interpretation of (external) sensory input in the CNS. Due to the observed atypicalities in ANS activation, this ‘filter’ is speculated to be deviant among individuals with ASD. If, by default, ANS reacts to sensory input with heightened arousal (i.e., hyperarousal) that stimulus may be interpreted as a threat leading to avoidance behaviour or aggression. On the other hand, if sensory input has only little or no effect on ANS activity (i.e., hypoarousal) that stimulus might not be regarded as salient resulting in underresponsiveness. In this systematic review we investigated whether the existing literature supports our speculation that the variation in psychophysiological state could explain both individual and moment-to-moment variation in processing and interpretation of social information.
Given the evidence for altered neural and physiological reactivity to social stimuli in ASD, our aim was to systematically review and qualitatively synthesise the evidence existing in support of or against our hypothesis of atypical (i.e., enhanced or decreased) body-brain interaction among individuals with ASD. Prior to the examination of ANS and CNS interactions, we first assembled the empirical evidence regarding coexisting differences in ANS and CNS activation during social information processing between individuals with ASD and typically developing individuals. Second, as our main question, we systematically reviewed the literature to investigate whether there is evidence that ANS-CNS interaction contributes to the processing of social information differently among individuals with ASD and typically developing individuals. To reach these aims we systematically reviewed studies that used simultaneous non-invasive measurement of ANS and CNS activity during social information processing among individuals with ASD and typically developing individuals.
Methods
This systematic review was conducted according to PRISMA guidelines (Moher et al., 2009). However, this study or its protocol were not preregistered.
Search Procedures
Comprehensive literature searches were conducted in five databases: PubMed, Medline, PsychInfo, PsychArticles, and Cinahl (until 12.1.2022). In all databases, search terms were inserted as free text into the search term field. Each search term consisted of a combination of keywords related to ASD, well-established non-invasive brain imaging methods and autonomic nervous system measures, such as (“autism” OR “autism spectrum disorder” OR “autistic” OR “Asperger”) AND (“MEG” OR “magnetoencephalography” OR “EEG” OR “encephalography” OR “fMRI” OR “functional magnetic resonance imaging”) AND (“SCR” OR “EDA” OR “skin conductance” OR “electrodermal” OR “galvanic skin response”).
ASD | autism, autism spectrum disorder, autistic, Asperger |
CNS | MEG, magnetoencephalography, EEG, electroencephalography, fMRI, functional magnetic resonance imaging |
ANS | ECG, EKG, HRV, heart, heart rate, heart rate variability, cardiac; respiration, respiratory, breathing; pupil, pupillary response, blink, eye tracking, gaze; SCR, EDA, skin conductance, electrodermal, galvanic skin response; autonomic nervous system, parasympathetic, sympathetic, physiological response |
Study Selection
First, all the titles and abstracts of studies identified through the systematic search were screened for initial inclusion. If sufficient information was not provided in the title and abstract, full-text articles were retrieved. Studies were included for further assessment if they were peer-reviewed studies written in English. In addition, inclusion required that the ASD population was examined, study design included processing of socially relevant information, such as social cues (e.g., eye contact) and emotional expressions (e.g., facial expression of sadness) that are crucial for social understanding and social behaviour, and both ANS and brain activity were measured noninvasively. Since an inclusive definition of body-brain interaction was emphasised, causality between ANS and CNS measures was not required. The year of publication was not restricted.
Studies were excluded if either ANS or CNS outcome was missing or there was no social element (e.g., social cues, social interaction, emotions, faces, gaze direction, and empathy) in the study design. Furthermore, studies without ASD populations (e.g., siblings of individuals with ASD, individuals with increased likelihood of ASD, and individuals with subclinical ASD) were excluded. Case reports, systematic reviews, reviews, meta-analyses, study protocols, comments, opinions, and method papers were discarded. After removing duplicates, the reference lists for studies meeting the initial inclusion criteria were reviewed to identify additional studies for possible inclusion, but no new articles were found. Finally, the included full-text articles were assessed for eligibility. The following eligibility criteria (PICOTS) were used:
-
1.
Population: individuals of all ages with a diagnosis of ASD
-
2.
Intervention/exposure: socially relevant stimulus
-
3.
Comparison: typically developing individuals
-
4.
Outcomes: autonomic and central nervous system activity measured simultaneously
-
5.
Time of publication: not restricted
-
6.
Study type: peer-reviewed original research paper written in English
Studies that did not meet the eligibility criteria were excluded from the qualitative synthesis.
Data Extraction
Studies meeting the eligibility criteria were summarised in terms of participant characteristics, experimental design, and outcome variables (i.e., ANS and CNS responses during social information processing). In addition, clinical information relevant for assessing the applicability and interpretation of the findings was extracted from the data. Thus, the following objective data was extracted from each study: gender and age of participants, the total number of participants in each group, criteria to match the ASD and comparison groups, information regarding ASD diagnosis (e.g., diagnostic criteria and methods), social stimuli used in the experiment, and measures of brain and ANS activity along with their outcomes. Where applicable, co-occurring conditions and the use of medication were also extracted to assess the homogeneity of comparison groups.
Quality Assessment
Since no tools existed directly applicable for quality assessment of non-interventional, cross-sectional (neuro)physiological studies, we created a quality assessment checklist in accordance with recommendations for the assessment of methodological quality standards (Farrington, 2003) and the quality assessment measure devised by Lydon et al. (2014). Furthermore, criteria specific to research involving individuals with ASD were developed based on the reviews by Jarrold and Brock (2004) and Cohen et al. (2011).
The quality assessment checklist used to evaluate the studies included in this systematic review consists of 12 criteria that are separated into five categories: descriptive validity, internal validity, external validity, construct validity, and statistical conclusion validity (Farrington, 2003). Each criterion is scored 0 – 2 and defined either as high (2 = criterion is met), moderate (1 = criterion is partially met), or low (0 = criterion is not met) depending on the degree to which each criterion is met. Detailed description of the criteria and the assessment of the methodological quality of each study is represented in the Supplementary Material.
Reliability of Search Procedures and Inter-Rater Agreement
To ensure the accuracy and reliability of the systematic literature search, authors 1 and 3 determined the detailed search terms, initial inclusion criteria and eligibility criteria. Then author 1 conducted the systematic literature searches and screened the articles for initial inclusion. Full-text review and assessment for eligibility were conducted independently by authors 1 and 3. Agreement on the eligibility of the articles was obtained on 28 of the 30 studies, that is, 93%. Disagreement occurred because the two studies were included by one author and excluded by the other. The agreement was achieved through consultation with author 2. Thereafter, author 1 extracted the relevant information from each eligible article. Similarly, the quality assessment of included studies was carried out by author 1. The accuracy of data extraction and appraisal of the studies was assessed by authors 2 and 3. If non-agreement emerged, consensus between authors was achieved through discussion and the data extracted and the appraisal of the study was revised accordingly.
Results
Studies Identified
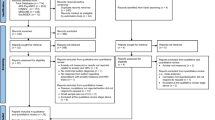
Overall, the systematic literature searches identified 1892 studies (see Fig. 1). After screening the titles and abstracts for initial inclusion criteria, irrelevant studies were excluded (n = 1841) and duplicates were removed (n = 21). Thus, 30 full-text articles were further examined in detail to assess for eligibility. Twenty-four studies were excluded with the following reasons: missing an outcome measure or CNS and ANS activity were measured separately (n = 19); no comparison group as a comparison (n = 1); missing an outcome measure and no comparison group (n = 2); no social stimuli, missing an outcome measure and no comparison group (n = 1); and no social stimuli and ineligible study type (n = 1). Eventually, six studies fulfilled the eligibility criteria and were included in the qualitative synthesis.

Adapted from Moher et al. (2009)
PRISMA flow diagram for systematic review literature search.
Sample Characteristics
Participant and study characteristics are summarised in Table 1. The year of publication ranged from 2005 to 2015. Included studies were conducted in Finland, Germany, the Netherlands, and the USA. The sample sizes ranged from 29 to 62. In total, the eligible studies included 248 participants, of which 128 were individuals with ASD and 120 were typically developing individuals. Furthermore, 97.2% of the participants were male and 2.8% were female. Two of the studies investigated children (Kylliäinen et al., 2012; Van Hecke et al., 2009), one study focused on teenagers (Dalton et al., 2005), and the remaining three studies involved adults (Althaus et al., 2015; Gu et al., 2015; Krach et al., 2015) with and without ASD.
In all six studies, clinical ASD diagnoses were confirmed using Autism Diagnostic Interview-Revised or Autism Diagnostic Observation Schedule. Three studies reported that participants with ASD met the diagnostic criteria described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Althaus et al., 2015; Dalton et al., 2005; Gu et al., 2015), and one study reported meeting the criteria described both in DSM-IV and in International Classification of Diseases, Tenth Revision (Krach et al., 2015). The remaining two studies (Kylliäinen et al., 2012; Van Hecke et al., 2009) did not specify the criteria for the clinical ASD diagnosis. All of the participants with ASD had either Full Scale Intelligence Quotient (IQ) ≥ 75, Verbal IQ or Performance IQ within normal range. Cognitive assessment was conducted using Wechsler Adult Intelligence Scale (WAIS-III, WAIS-R), Wechsler Intelligence Scale for Children (WISC-III, WISC-IV), Groninger Intelligentie Test, Kaufman Brief Intelligence Test, Wide Range Intelligence test or Stanford-Binet test for general IQ. ASD and comparison groups were matched for age and sex in five studies. In addition, four studies matched the comparison group for intelligence (Althaus et al., 2015; Gu et al., 2015; Krach et al., 2015; Kylliäinen et al., 2012), and one study also considered the socioeconomic status (Gu et al., 2015).
Only one study reported that the participants were taking medication (Van Hecke et al., 2009). Two studies stated that the participants were free from the use of psychoactive and neuroleptic medication (Althaus et al., 2015; Gu et al., 2015). The use of medication was not reported in three studies (Dalton et al., 2005; Krach et al., 2015; Kylliäinen et al., 2012). In all six studies, comparison participants were healthy, typically developing individuals with no psychiatric or developmental diagnosis. Only one study reported using neurological and psychiatric conditions as exclusion criteria for individuals with ASD (Gu et al., 2015), otherwise, co-occurring conditions were not reported.
Experimental Designs
As indicators of ANS activation, two studies used heart-rate related measures, that is, the maximum cardiac deceleration and RSA (Althaus et al., 2015; Van Hecke et al., 2009), and another two studies used eye-tracking measures, that is, pupil diameter and fixations (Dalton et al., 2005; Krach et al., 2015). Moreover, SCRs were measured in two studies (Gu et al., 2015; Kylliäinen et al., 2012). Since pupil diameter, fixation patterns and SCR are regarded as measures of sympathetic activity and maximum cardiac deceleration and RSA are considered measures of parasympathetic activity, most of the studies measured the sympathetic division of ANS (Dalton et al., 2005; Gu et al., 2015; Krach et al., 2015; Kylliäinen et al., 2012) and the remaining two studies measured the parasympathetic division (Althaus et al., 2015; Van Hecke et al., 2009). CNS activation was measured using electroencephalography in three studies (Althaus et al., 2015; Kylliäinen et al., 2012; Van Hecke et al., 2009) and functional magnetic resonance imaging in the remaining three studies (Dalton et al., 2005; Gu et al., 2015; Krach et al., 2015).
Social stimuli used in the experiments (see Table 2) included affective pictures from the International Affective Picture System (IAPS) database with and without humans (Althaus et al., 2015), images of emotional and neutral faces (Dalton et al., 2005), images of familiar and unfamiliar faces (Dalton et al., 2005; Kylliäinen et al., 2012), videos of familiar and unfamiliar people (Van Hecke et al., 2009), as well as images depicting socially (Krach et al., 2015) and physically painful and non-painful situations (Gu et al., 2015; Krach et al., 2015). Furthermore, Gu et al. (2015) and Krach et al. (2015) used partly similar pictures as physically painful stimuli in their experiments. Most of the studies were conducted using static images, but one study used videos (Van Hecke et al., 2009) and another study used static pictures that were ‘looming’ towards the participant (Kylliäinen et al., 2012).
Only one study reported measuring baseline activity (i.e., resting state measurement in the absence of any task or stimulation) separately from stimulus presentation (Van Hecke et al., 2009), otherwise baseline-adjusted ANS and CNS measures were used for data analysis, except in the study by Dalton et al. (2005) in which no baseline-adjustment was used. Three of the studies used non-social stimuli as a control condition; pictures with scenes (Althaus et al., 2015), videos of objects (Van Hecke et al., 2009), and images of cars (Kylliäinen et al., 2012). Moreover, Dalton et al. (2005) used images of familiar and unfamiliar objects as a control condition in facial recognition task, but the results were not discussed in the article. Additionally, the use of a control condition was not reported in the emotion discrimination task (Dalton et al., 2005). Furthermore, in two studies, the use of a non-social control condition was not reported (Gu et al., 2015; Krach et al., 2015). No differences in ANS or CNS activation were found between individuals with ASD and typically developing individuals in the non-social control conditions (Althaus et al., 2015; Kylliäinen et al., 2012; Van Hecke et al., 2009).
Results of Individual Studies
ANS activation during social information processing
The main findings of each study are summarised in Table 3. When examining the ANS responses to social stimuli, three of the studies reported higher levels of arousal, that is, hyperarousal in individuals with ASD compared with the typically developing individuals (Dalton et al., 2005; Gu et al., 2015; Van Hecke et al., 2009). One study, in turn, reported lower levels of arousal, that is, hypoarousal in the ASD group (Kylliäinen et al., 2012) and another found no significant differences between the ASD and typically developing (TD) groups in ANS reactivity to social stimuli (Althaus et al., 2015). Furthermore, the study by Krach et al. (2015) found hypoarousal in social pain task and no group differences in physical pain task. Hyperarousal was reflected as lower RSA to videos of unfamiliar people (Van Hecke et al., 2009), atypical fixation patterns to images of faces (Dalton et al., 2005) and enhanced SCR to physically painful images (Gu et al., 2015). Hypoarousal, in turn, was reflected as smaller pupil diameter to images depicting social pain (Krach et al., 2015) and attenuated SCR to images of faces (Kylliäinen et al., 2012).
CNS activation during social information processing
Differences in CNS responses to social stimuli between individuals with ASD and typically developing individuals were found in four studies, whereas two studies reported no group differences (Althaus et al., 2015; Van Hecke et al., 2009). Krach et al. (2015) reported no group differences in hemodynamic brain responses when images depicting vicarious physical pain were presented, but they also reported less pronounced brain activation overall and specific decreases in AIC and ACC to images depicting vicarious social pain among individuals with ASD. Kylliäinen et al. (2012) reported no approach-related, that is, relative left-sided frontal electrophysiological brain activation to images of faces in the ASD group that was observed in the typically developing individuals. The remaining two studies found both specific increases and decreases in CNS activation to social stimuli. Dalton et al. (2005) showed greater hemodynamic activation in amygdala and orbitofrontal gyrus along with less activation in the fusiform gyrus (FG), occipital gyrus and middle frontal gyrus during emotion discrimination among individuals with ASD. Greater activation was found in the amygdala and less activation in FG and occipital cortex during facial recognition. Gu et al. (2015) showed greater hemodynamic activation in AIC and extrastriate body area along with decreased activation in lateral PFC to physically painful images in the ASD group. Although Gu et al. (2015) and Krach et al. (2015) used similar pictures depicting physical pain, their findings were contradictory. Furthermore, opposing findings regarding AIC activation, that is decreased AIC activation during vicarious social pain task and increased AIC activation during physical pain task, were reported (Gu et al., 2015; Krach et al., 2015). Considering the experimental settings, these opposing findings could be explained by the differences in the task demands as participants were either judging whether the individual in the image was suffering from pain or not (Gu et al., 2015) or estimating the intensity of physical and social pain depicted in the image (Krach et al., 2015).
Interaction between ANS and CNS during social information processing
The association between ANS and CNS measures was not directly investigated in two studies (Althaus et al., 2015; Kylliäinen et al., 2012). Van Hecke et al. (2009) reported that RSA and temporal-parietal brain activation were not associated in either of the groups when videos of familiar and unfamiliar people were presented. Similarly, Krach et al. (2015) found no association between pupil dilation and hemodynamic responses in AIC and anterior cingulate gyrus (ACG) in the ASD group when images depicting vicarious social pain were represented, although the variability in the pupil dilation covaried with the dynamics in hemodynamic responses in the comparison group. Furthermore, both ASD and TD groups showed similar covariation between pupil diameter and hemodynamic responses to images depicting vicarious physical pain (Krach et al., 2015). Dalton et al. (2005) and Gu et al. (2015), in turn, reported positive associations between ANS and CNS measures. Dalton et al. (2005) showed that activation in the amygdala and FG were strongly and positively associated with the amount of time spent fixating on the eyes in the ASD group, but not in the comparison group. Furthermore, Gu et al. (2015) reported an association between SCR and activity in the AIC in both groups. It is worth noting that the correlation between these measures was enhanced in the ASD group in comparison with the comparison group.
Although using partly similar pictures depicting physical pain, Gu et al. (2015) reported an enhanced association between ANS and CNS among individuals with ASD, whereas Krach et al. (2015) reported that the interaction between ANS and CNS was similar in both groups. Moreover, Krach et al. (2015) found no association between ANS and CNS during vicarious social pain task among individuals with ASD. These somewhat contradictory findings may be explained by the differences in task demands, as mentioned earlier, because judging whether someone is suffering pain or not and estimating the intensity of pain (physical pain or social embarrassment) may involve critically different processes and neural networks. Moreover, the vicarious physical and social pain tasks used in the study by Krach et al. (2015) required taking another person’s perspective. Thus, also the level of cognitive and bodily engagement may have differed between these tasks resulting in the observed differences. Furthermore, the brain regions of interest (excl., AIC) differed between these studies, which may also explain these opposing findings.
Associations between ANS or CNS activation and participant characteristics
Associations between ANS or CNS activation and participant characteristics were investigated in three studies. Dalton et al. (2005) reported a marginal correlation between neural activation and IQ for the ASD group, otherwise no significant correlations were found. Krach et al. (2015), reported that in the ASD group activation within the AIC during social pain task, but not during physical pain task, was inversely related to autism symptom severity in the domain of social affect (ADOS). Van Hecke et al (2009) found no significant correlations between IQ and physiological data, whereas higher baseline RSA correlated with higher levels of social skills and lower levels of problem behaviours on the Social Skills Rating System (SSRS), as well as lower (i.e., more typical) social communication scores, autistic mannerism scores, and total scores on the Social Responsiveness Scale (SRS) when the whole sample was investigated. Moreover, higher baseline alpha activity measured with EEG was related to lower (i.e., more typical) social motivation scores. When groups were tested separately, no significant correlations were found in the group of typically developing individuals. In the ASD group, higher baseline RSA was correlated with higher levels of social skills on the SSRS, and higher baseline alpha activation was related to lower (i.e., more typical) social motivation scores. These results imply that the activation of ANS or CNS may, at least partly, reflect the heterogeneity of individual characteristics observed among individuals with ASD.
Methodological Quality
The summary of the assessment of methodological quality is presented in Fig. 2. Overall, the methodological quality of the eligible studies included in this systematic review was adequate (from moderate to high). Limitations mainly concerned the descriptive validity of the studies, such as small sample sizes (6/6 studies), insufficient description of participant characteristics (5/6 studies) as well as inclusion and exclusion criteria (5/6 studies). Moreover, the internal validity in most of these studies was compromised due to the lack of baseline measurements of ANS and CNS activity (5/6 studies). In turn, statistical conclusion validity, construct validity and external validity were high across all the studies. For instance, operational definitions of the outcome measures (5/6 studies), representativeness of the stimuli (4/6 studies), appropriate reporting of analysis methods (4/6 studies) and missing data (5/6 studies) as well as the investigation of statistical significance (6/6 studies) were mainly of high quality.
Discussion
Summary of the Findings
The aim of this article was to systematically review and qualitatively synthesize the empirical evidence regarding differences in ANS and CNS activation as well as body-brain interaction during social information processing between individuals with ASD and typically developing individuals. The focus was on studies where both ANS and CNS activity were simultaneously measured. First, differences in ANS and CNS activations between individuals with ASD and typically developing individuals were examined separately. As expected, based on the previous literature (e.g., Lydon et al., 2014), both ANS hypoarousal (attenuated SCR, smaller pupil diameter) and hyperarousal (enhanced SCR, lower RSA, atypical eye fixation pattern) were observed during processing of social information among individuals with ASD. Moreover, two of the studies found no differences in autonomic arousal between typically developing individuals and individuals with ASD (Althaus et al., 2015; Krach et al., 2015 [physical pain]).
Atypicalities in the CNS activation during social information processing were also reported among individuals with ASD. Differences were found in the hemodynamic activation of amygdala, AIC, and ACC (Dalton et al., 2005; Gu et al., 2015; Krach et al., 2015 [social pain]), which are considered relevant in processing of social information (Van Overwalle, 2009). Moreover, Kylliäinen et al. (2012) demonstrated no approach-related electrophysiological brain activation that was observed among the typically developing individuals. However, there were also studies indicating similar brain activation in ASD and TD groups during social information processing (Althaus et al., 2015; Krach et al., 2015 [physical pain]; Van Hecke et al., 2009). Taken together, these observations provide initial evidence regarding both ANS and CNS atypicalities during processing of socially relevant information among some individuals with ASD, although the results are still inconsistent and context dependent.
As our main question we investigated whether the interaction between ANS and CNS is meaningful in understanding ASD, in other words whether ANS-CNS interaction contributes to the processing of social information differently among individuals with ASD and typically developing individuals. The interaction between ANS and CNS activation was not directly approached in two studies (Althaus et al., 2015; Kylliäinen et al., 2012) and one study (Van Hecke et al., 2009) reported no association between cardiac and electric brain activation patterns in either of the groups. In the remaining three studies, atypicalities in interaction between ANS and CNS were observed among individuals with ASD. The association between ANS and CNS during emotion discrimination and facial recognition was observed only among individuals with ASD, and not among typically developing individuals (Dalton et al., 2005). Based on these findings, Dalton et al. (2005) claim that atypical eye fixation pattern is associated with overarousal and further, the face-processing deficits in ASD result from hyperactivation in the central circuitry of emotion leading to heightened sensitivity to social stimuli.
When investigating empathy for physical pain, enhanced interaction between ANS and CNS activation in individuals with ASD were demonstrated (Gu et al., 2015). Thus, it was proposed that the heightened autonomic and cortical arousal might result in empathy deficits in ASD (Gu et al., 2015). Findings by Krach et al. (2015), in turn, suggested that the embodied representation of complex emotions such as empathy for social pain is reduced among individuals with ASD in comparison with the typically developing individuals, but basic abilities related to sharing another’s affect remain intact. Based on these findings, it can be speculated that among individuals with ASD the contribution of ANS on neural processing of empathy for physical pain is similar or even enhanced in comparison with typically developing individuals. However, there is no interaction between ANS and CNS during processing of vicarious social pain among individuals with ASD. Although the small number of studies does not allow to make strong conclusions, the integration of the above findings would allude that there indeed seems to be an altered contribution of ANS-CNS interaction among individuals with ASD during processing of socially relevant information, but more evidence is needed to understand this body-brain interaction, and especially the causal relations, in social contexts more accurately.
Altogether, our ultimate aim was to synthesize the evidence existing in support of or against our hypothesis of atypical body-brain interaction among individuals with ASD during social information processing. Based on the fairly limited evidence, interaction between the ANS and CNS appears to be enhanced in ASD (Dalton et al., 2005; Gu et al., 2015). This was demonstrated between fixation patterns and hemodynamic responses, as well as SCR and hemodynamic responses during processing of images of faces and physically painful situations, respectively. On the contrary, ASD was characterized with diminished interaction between ANS and CNS in response to socially painful situations (Krach et al., 2015). Thus, tentative empirical evidence exists in support of our hypothesis of atypical (i.e., enhanced or decreased) body-brain interaction among individuals with ASD during processing of socially relevant information.
On the contrary, no association between RSA and spectral alpha band power in either of the groups was found when videos of familiar and unfamiliar people were presented, thus not supporting our assumption but not rejecting it either (Van Hecke et al., 2009). However, this contradictory finding may be explained by factors related to the experimental setting. Since videos were used as social stimuli (Van Hecke et al., 2009), whereas other studies used static images, the complexity and ecological validity of the videos (incl., listening to a story, familiar and unfamiliar readers, naturalistic gestures, direct eye gaze) may have affected the findings. Additionally, watching a video may be more activating overall leading to differential activation patterns in comparison with static images. Furthermore, Van Hecke et al. (2009) only investigated right temporal-parietal activity, whereas the possible associations between ANS and CNS could have been localized to other brain areas (cf., Beissner et al., 2013). Therefore, it is possible that the CNS measure used was not optimal to investigate body-brain interaction. Taken together, the systematic review of the literature mainly supports our speculation of atypical body-brain interaction among individuals with ASD, but there is also evidence against our speculation.
Limitations and Recommendations for Future Research
The results of this systematic review must be considered in the context of several limitations. First, the extensive literature search identified over 1800 studies but only six of them met the eligibility criteria, indicating that there is plenty of research regarding ANS and CNS activation, but only a few studies have investigated ANS and CNS activation simultaneously during social information processing. Thus, our literature search was rather inclusive and may therefore be criticized for the lack of specificity. However, our aim was to ensure that as many relevant studies as possible were included in the systematic review and hence the sensitivity of the literature search was emphasised. It must also be noted that due to the restrictions regarding the small number of included studies and the heterogeneity of the findings, conducting a meta-analysis was not reasonable.
One of the main limitations in this systematic review was that the sample sizes in each study were relatively small. Moreover, most of the studies only involved individuals with ASD that had either FSIQ ≥ 75 or Verbal or Performance IQ within the normal range. Since majority (97.2%) of the participants in the included studies were male, these results may not be applicable for females or non-binary people. Thus, there is a risk of bias in this systematic review and the results cannot be generalised to the entire autism spectrum as such. In the future, studies with larger and more representative samples are needed.
In addition, there was high variability in the results across the studies included in this systematic review that may be explained by several factors. First, different combinations of ANS and CNS measures (e.g., RSA – EEG and pupillometry – fMRI) were used in these studies. Consequently, since SCR, pupillometry and fixation patterns mainly reflect sympathetic activity and maximum cardiac deceleration and RSA are considered mainly parasympathetic, some studies of the present review measured the sympathetic division of ANS (i.e., SNS), whereas others focused on the parasympathetic branch (i.e., PSNS). Furthermore, there were differences across studies in the neural features investigated (e.g., hemodynamic responses or electric activation with focus on time- or frequency-domain characteristics and differences in the brain regions of interest). Moreover, some of the interpretations given to the selected ANS and CNS measures are highly context dependent or not well-established. For instance, while most of the studies used direct measures of ANS activation, sometimes also indirect derivative measures, such as fixations patterns, were used (Dalton et al., 2005). Typically, eye-tracking is used to ensure similar viewing patterns between comparison groups and to control for the influence of viewing pattern on brain activation (e.g., Greene et al., 2011; von dem Hagen et al., 2014). Dalton et al. (2005) regarded atypical eye fixations as an indirect measure of ANS activation reflecting overarousal mediated by activation of limbic areas and not just differential pattern of scanning faces. Furthermore, some of the used brain imaging measures, such as frontal asymmetry (Kylliäinen et al., 2012) have been considered inconsistent and, to some extent, controversial (e.g., Smith et al., 2017).
Second, a wide variety of tasks and social stimuli were used, so the variation in task demands, cognitive effort and emotional aspects of the experimental designs may explain the variability in the results. Furthermore, tasks and stimuli also varied in the degree to which they can capture the complexity and authenticity of social information processing in the real world. Taking authenticity into consideration is highly important, because previous studies have found differences in autonomic and neural responses to socially relevant stimuli when comparing static pictures, videos, and live interaction (Hietanen et al., 2020; Pönkänen et al., 2011). It is also worth mentioning that none of the included studies used natural social communication or interaction as a stimulus or task. These kinds of natural experimental settings are shown to be feasible (e.g., Karvonen et al., 2016; Silvennoinen et al., 2022; Stevanovic et al., 2019) and would be essential in demonstrating whether ANS-CNS interaction influences the day-to-day social functioning among individuals with ASD. Although the high variability in experimental settings is beneficial for developing novel methodological approaches, standardised way of conducting studies is required for replicating previous findings and increasing our understanding of ASD.
Third, it is possible that the variability in the results reflects the heterogeneity of individual characteristics across the autism spectrum. Even though criteria for matching (e.g., age, gender, IQ) were well reported in each study, the exclusion criteria were vaguely defined. For instance, the use of medication was not systematically reported. Only one study (Gu et al., 2015) reported using neurological and psychiatric conditions as exclusion criteria for participants with ASD, but otherwise co-occurring conditions were not explicitly reported. Thus, assessing the possible confounding factors such as co-occurring developmental (e.g., attention-deficit/hyperactivity disorder, sensory processing disorder, specific language impairment), mental health (e.g., depression, anxiety, obsessive–compulsive disorder) and physical conditions (e.g., cardiovascular disease) or personality traits (e.g., alexithymia), was not possible. Since these background factors may also add complexity to understanding the body-brain interaction across autism spectrum, they should be either excluded in advance or eventually controlled for using statistical analysis approaches (Jarrold & Brock, 2004; Lydon et al., 2014).
Due to the high heterogeneity of ASD, it is important that participant characteristics are described also beyond the diagnosis (e.g., detailed description of social functioning, communication skills and sensory processing), enabling more accurate comparison of results between studies and evaluation of generalisability of the results across the autism spectrum. Alternatively, recruiting a group of individuals with a specific trait related to social functioning would allow investigating how body-brain interaction is associated with certain social information processing features among individuals with ASD. Furthermore, to take neurodiversity into account, recruiting both well-matched typically developing individuals and several comparison groups, such as individuals with other developmental or neuropsychiatric conditions, would be beneficial in establishing the variance of the autonomic and neurophysiological responses during social information processing. Taken together, due to the small number of eligible studies included in this systematic review, as well as high variability in physiological and neural measures, social stimuli and sample characteristics, the conclusions of this systematic review need to be interpreted with caution.
Despite rapidly accumulating empirical evidence, the knowledge and understanding of the underlying neural and physiological mechanisms associated with ASD have not improved at the same pace due to the lack of replicability. Notably, the variability across studies in applied measures, task demands and stimuli, was observed in this systematic review as well. Hence, a clear shortcoming in the reviewed studies, from the perspective of our research question, is the lack of systematic protocol for using simultaneous ANS and CNS recordings. It is worth noting that although the synthesis of the results suggests atypical body-brain interaction among individuals with ASD, the involved studies were originally designed for different purposes. Thus, the methodological choices may not have been optimal for investigating the interaction between ANS and CNS during social information processing. To examine body-brain interaction in a systematic manner and to improve our understanding of social information processing among individuals with ASD in the future, a consistent standardised way of conducting and reporting (neuro)physiological research is needed. Here we suggest key notions that would help to harmonize the experimental settings when examining the interaction between ANS and CNS activation during processing of socially relevant information among individuals with ASD:
-
The use of existing standardised guidelines for conducting and reporting neurophysiological research (e.g., Camm et al., 1996; Gross et al., 2013) would enable quality control and reproducibility of studies. The lack of standardised practices in conducting and reporting neurophysiological research involving individuals with ASD has been pointed out in several reviews (Lydon et al., 2014; Patriquin et al., 2019), also including our systematic review.
-
Correct selection, measurement, and interpretation of specific ANS recordings along with detailed reporting of used measures and analysis pipelines is needed to improve the accuracy and replicability of the results. The quality and specific features of the raw signal (e.g., length of recording, sampling rate, and number of ectopic heartbeats) influence the validity and accuracy of ANS outcome measure computation and interpretation.
-
When combining ANS and CNS measures, methodological restrictions specific to the brain imaging modality need to be acknowledged. For instance, physiological recordings can be effortlessly combined with EEG and MEG, while with fMRI they need specific technical considerations to ensure safety and quality of the data (Babiloni et al., 2009; Bulte & Wartolowska, 2017; Gray et al., 2009; Iacovella & Hasson, 2011).
-
Establishing the baseline variance is necessary to determine whether the atypical characteristics observed in ASD reflect trait-like differences (i.e., differences in resting ANS or CNS activity) instead of state-like differences (i.e., differences in reactivity to stimuli or task induced conditions). Additionally, a non-social control condition would allow to assess specificity of possible differences in social tasks.
-
The use of standardised stimulus (e.g., pictures, videos, and other pre-prepared stimuli) sets or well-controlled authentic social settings is recommended to increase the cross-study interpretation (see e.g., IAPS database; Lang et al., 2008). When applying experimental settings involving natural social interaction, detailed description of methods and experimental setting is important. The complexity and authenticity of the stimuli and tasks need to be considered when reporting and interpreting the results.
-
Acknowledging individual variability and taking into account individual differences as well as intervening factors across the autism spectrum, instead of considering individuals with ASD as one group, facilitates cross-study comparison and increases the generalisability of the results.
To establish understanding on the role of ANS driven alterations in ASD, future studies that examine the relevance of body-brain interaction during social information processing would benefit from more strongly building on existing theories on the role of ANS in human experience and ANS-CNS interaction (e.g., Critchley & Harrison, 2013). It would also be essential to conduct experiments that directly test whether and how ANS activation modulates the processing of social information in the CNS among individuals with ASD and typically developing individuals. It is particularly challenging to go beyond correlative evidence to establish causal relations. For this, one approach is to modify the state of ANS, for example using relaxation techniques, medication, or even direct stimulation of the vagus nerve, and assess the modulatory effects of ANS both on the neural and behavioural level (Khalsa et al., 2018). Alternatively, experimental designs in which stimuli are presented during different phases of ANS rhythms (e.g., inspiration vs. expiration, cardiac systole vs. diastole) could be used in investigating body-brain-cognition coupling (Parviainen et al., 2022).
If our primary hypothesis on body-brain interaction is correct and ANS activation indeed modulates CNS activity and further the perception and interpretation of social information, testing and developing interventions that affect ANS activity (e.g., breathing and relaxation techniques, sensory stimulation, and animal-assisted therapy) could be beneficial. Especially, understanding how ANS reactions are linked with processing and interpretation of socially relevant information could increase self-knowledge and self-esteem of individuals with difficulties in social interaction. Moreover, improving self-regulation by learning to regulate ANS activation could support social functioning as well as wellbeing and participation of individuals with ASD. In the future, studies examining how body-brain interaction during development gives rise to characteristics associated with autism are needed. Furthermore, it would be of great importance to understand how genetic, environmental, and individual factors (e.g., temperament, interoceptive awareness and sensitivity, sensory processing atypicalities) influence the manifestation of body-brain interaction throughout the lifespan.
From the methodological point of view, a greater focus on combining two or more measures of ANS activation would also improve our understanding regarding the interplay between PSNS, SNS and CNS during processing of socially relevant information. Indeed, each recording (e.g., EDA, HRV, pupillometry) alone captures a limited view on the ANS functions typically emphasising either sympathetic or parasympathetic division. In general, acknowledging the rich and complementary information available in different ANS recordings is likely to increase our understanding of the role of bodily states for experience. Moreover, future methodological advancements in mobile EEG, MEG, and functional near-infrared spectroscopy as well as in hyperscanning enables to test body-brain interaction also in ecologically valid experimental settings, such as during natural social interaction. The studies conducted in natural settings should, however, be accompanied by studies, where the parameters can be strictly controlled and manipulated to achieve reliable interpretation for the different features reflecting ANS-CNS coupling.
In the future, research assessing the influence of individual factors, such as gender, co-occurring conditions, and personality traits, could clarify the individual variability in body-brain interaction. Since there is a strong gender bias towards clinical presentation of ASD in males (Loomes et al., 2017), future studies should take gender bias as well as gender diversity into account. Furthermore, the use of several comparison groups would enable studying possible transdiagnostic mechanisms and processes as well as diagnosis-specific phenomena.
Conclusions
In summary, the results of this systematic review demonstrate coexisting but context dependent ANS and CNS atypicalities during processing of socially relevant information among individuals with ASD. Furthermore, there is indication of altered reactivity and/or trait features in ANS activity among individuals with ASD that may contribute to social information processing by influencing the perception and processing of socially relevant stimuli in the brain. However, more empirical evidence is needed to establish our knowledge of the body-brain interaction and its role in social functioning among individuals with ASD. Notably, standardised research practices and rigorous use of methods should be fostered to achieve reliable increment of knowledge regarding the underlying neural and physiological mechanisms contributing to alterations in social functioning and social information processing. Furthermore, understanding how individuals with ASD process socially relevant information is crucial for development of support services and interventions that aim at improving the well-being and participation of individuals with ASD.
Data Availability
All the studies included in this systematic review are publicly available.
References
Acharya, R. U., Joseph, P. K., Kannathal, N., Lim, C. M., & Suri, J. S. (2006). Heart rate variability: A review. Medical & Biological Engineering & Computing, 44, 1031–1051. https://doi.org/10.1007/s11517-006-0119-0
Althaus, M., Groen, Y., Wijers, A. A., Noltes, H., Tucha, O., & Hoekstra, P. J. (2015). Oxytocin enhances orienting to social information in a selective group of high-functioning male adults with autism spectrum disorder. Neuropsychologia, 79, 53–69. https://doi.org/10.1016/j.neuropsychologia.2015.10.025
Amaral, D. G., Schumann, C. M., & Nordahl, C. W. (2008). Neuroanatomy of autism. Trends in Neurosciences, 31, 137–145. https://doi.org/10.1016/j.tins.2007.12.005
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (5th ed.). American Psychiatric Association.
Ammons, C. J., Winslett, M. E., & Kana, R. K. (2021). Neural responses to viewing human faces in autism spectrum disorder: a quantitative meta-analysis of two decades of research. Neuropsychologia, 150, 107694. https://doi.org/10.1016/j.neuropsychologia.2020.107694
Arora, I., Bellato, A., Ropar, D., Hollis, C., & Groom, M. J. (2021). Is autonomic function during resting-state atypical in Autism: A systematic review of evidence. Neuroscience and Biobehavioral Reviews, 125, 417–441. https://doi.org/10.1016/j.neubiorev.2021.02.041
Babiloni, C., Pizzella, V., Gratta, C. D., Ferretti, A., & Romani, G. L. (2009). Fundamentals of electroencefalography, magnetoencefalography, and functional magnetic resonance imaging. International Review of Neurobiology, 86, 67–80. https://doi.org/10.1016/S0074-7742(09)86005-4
Badcock, P. B., Davey, C. G., Whittle, S., Allen, N. B., & Friston, K. J. (2017). The depressed brain: An evolutionary systems theory. Trends in Cognitive Sciences, 21, 182–194. https://doi.org/10.1016/j.tics.2017.01.005
Bal, E., Harden, E., Lamb, D., Van Hecke, A. V., Denver, J. W., & Porges, S. W. (2010). Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40, 358–370. https://doi.org/10.1007/s10803-009-0884-3
Beissner, F., Meissner, K., Bär, K. J., & Napadow, V. (2013). The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. Journal of Neuroscience, 33, 10503–10511. https://doi.org/10.1523/JNEUROSCI.1103-13.2013
Berntson, G.G., Cacioppo, J.T. (2004). Heart rate variability: stress and psychiatric conditions, in: Malik, M., Camm, J. (Eds.), Dynamic Electrocardiography. Blackwell Publishing, Oxford, pp. 57–64. https://doi.org/10.1002/9780470987483.ch7
Berntson, G. G., & Khalsa, S. S. (2021). Neural circuits of interoception. Trends in Neurosciences, 44, 17–28. https://doi.org/10.1016/j.tins.2020.09.011
Bujnakova, I., Ondrejka, I., Mestanik, M., Visnovcova, Z., Mestanikova, A., Hrtanek, I., Fleskova, D., Calkovska, A., & Tonhajzerova, I. (2016). Autism spectrum disorder is associated with autonomic underarousal. Physiological Research, 65, S673–S682. https://doi.org/10.33549/physiolres.933528
Bulte, D., & Wartolowska, K. (2017). Monitoring cardiac and respiratory physiology during FMRI. NeuroImage, 154, 81–91. https://doi.org/10.1016/j.neuroimage.2016.12.001
Camm, A. J., Malik, M., Bigger, J. T., Breithardt, G., Cerutti, S., Cohen, R. J., Coumel, P., Fallen, E. L., Kennedy, H. L., Kleiger, R. E., Lombardi, F., Malliani, A., Moss, A. J., Rottman, J. N., Schmidt, G., Schwartz, P. J., & Singer, D. H. (1996). Heart rate variability: Standards of measurement, physiological interpretation and clinical use. European Heart Journal, 17, 354–381.
Candia-Rivera, D., Catrambone, V., Thayer, J. F., Gentili, C., & Valenza, G. (2022). Cardiac sympathetic-vagal activity initiates a functional brain–body response to emotional arousal. Proceedings of the National Academy of Sciences of the United States of America, 119, e2119599119. https://doi.org/10.1073/pnas.2119599119
Cheng, Y. C., Huang, Y. C., & Huang, W. L. (2020). Heart rate variability in individuals with autism spectrum disorders: A meta-analysis. Neuroscience and Biobehavioral Reviews, 118, 463–471. https://doi.org/10.1016/j.neubiorev.2020.08.007
Cohen, I.L., Yoo, J.H., Goodwin, M.S., Moskowitz, L. (2011). Assessing challenging behaviors in Autism Spectrum Disorders: Prevalence, rating scales, and autonomic indicators. In: Matson, J., Sturmey, P. (Eds.) International handbook of autism and pervasive developmental disorders. Autism and Child Psychopathology Series. Springer, New York, NY, pp. 247–270.https://doi.org/10.1007/978-1-4419-8065-6_15
Craig, A. D. (2014). How do you feel? An interoceptive moment with your biological self. Princeton University Press.
Critchley, H. D., & Garfinkel, S. N. (2018). The influence of physiological signals on cognition. Current Opinion in Behavioral Sciences, 19, 13–18. https://doi.org/10.1016/j.cobeha.2017.08.014
Critchley, H. D., & Harrison, N. A. (2013). Visceral influences on brain and behavior. Neuron, 77, 624–638. https://doi.org/10.1016/j.neuron.2013.02.008
D’Hondt, F., Lassonde, M., Collignon, O., Dubarry, A. S., Robert, M., Rigoulot, S., Honoré, J., Lepore, F., & Sequeira, H. (2010). Early brain-body impact of emotional arousal. Frontiers in Human Neuroscience, 4, 33. https://doi.org/10.3389/fnhum.2010.00033
Dalton, K. M., Nacewicz, B. M., Johnstone, T., Schaefer, H. S., Gernsbacher, M. A., Goldsmith, H. H., Alexander, A. L., & Davidson, R. J. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8, 519–526. https://doi.org/10.1038/nn1421
Damasio, A. R. (1998). Emotion in the perspective of an integrated nervous system. Brain Research Reviews, 26, 83–86. https://doi.org/10.1016/S0165-0173(97)00064-7
Di Martino, A., Ross, K., Uddin, L. Q., Sklar, A. B., Castellanos, F. X., & Milham, M. P. (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry, 65, 63–74. https://doi.org/10.1016/j.biopsych.2008.09.022
Dufour, N., Redcay, E., Young, L., Mavros, P. L., Moran, J. M., Triantafyllou, C., ... Saxe, R. (2013). Similar brain activation during false belief tasks in a large sample of adults with and without autism. PloS One 8, e75468. https://doi.org/10.1371/journal.pone.0075468
Eisenbarth, H., Chang, L. J., & Wager, T. D. (2016). Multivariate brain prediction of heart rate and skin conductance responses to social threat. Journal of Neuroscience, 36, 11987–11998. https://doi.org/10.1523/JNEUROSCI.3672-15.2016
Fan, Y. T., Chen, C., Chen, S. C., Decety, J., & Cheng, Y. (2014). Empathic arousal and social understanding in individuals with autism: Evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience, 9, 1203–1213. https://doi.org/10.1093/scan/nst101
Farrington, D. P. (2003). Methodological quality standards for evaluation research. The Annals of the American Academy of Political and Social Science, 587, 49–68. https://doi.org/10.1177/0002716202250789
Frith, C. D., & Frith, U. (2007). Social cognition in humans. Current Biology, 17, R724–R732. https://doi.org/10.1016/j.cub.2007.05.068
Galvez-Pol, A., McConnell, R., & Kilner, J. M. (2020). Active sampling in visual search is coupled to the cardiac cycle. Cognition, 196, 104149. https://doi.org/10.1016/j.cognition.2019.104149
Garfinkel, S. N., Minati, L., Gray, M. A., Seth, A. K., Dolan, R. J., & Critchley, H. D. (2014). Fear from the heart: Sensitivity to fear stimuli depends on individual heartbeats. Journal of Neuroscience, 34, 6573–6582. https://doi.org/10.1523/JNEUROSCI.3507-13.2014
Gray, M. A., Minati, L., Harrison, N. A., Gianaros, P. J., Napadow, V., & Critchley, H. D. (2009). Physiological recordings: Basic concepts and implementation during functional magnetic resonance imaging. NeuroImage, 47, 1105–1115. https://doi.org/10.1016/j.neuroimage.2009.05.033
Greene, D. J., Colich, N., Iacoboni, M., Zaidel, E., Bookheimer, S. Y., & Dapretto, M. (2011). Atypical neural networks for social orienting in autism spectrum disorders. NeuroImage, 56, 354–362. https://doi.org/10.1016/j.neuroimage.2011.02.031
Gross, J., Baillet, S., Barnes, G. R., Henson, R. N., Hillebrand, A., Jensen, O., Jerbi, K., Litvak, V., Maess, B., Oostenveld, R., Parkkonen, L., Taylor, J. R., van Wassenhove, V., Wibral, M., & Schoffelen, J. M. (2013). Good practice for conducting and reporting MEG research. NeuroImage, 65, 349–363. https://doi.org/10.1016/j.neuroimage.2012.10.001
Gu, X., Eilam-Stock, T., Zhou, T., Anagnostou, E., Kolevzon, A., Soorya, L., Hof, P. R., Friston, K. J., & Fan, J. (2015). Autonomic and brain responses associated with empathy deficits in autism spectrum disorder. Human Brain Mapping, 36, 3323–3338. https://doi.org/10.1002/hbm.22840
Hadjikhani, N., Joseph, R. M., Snyder, J., Chabris, C. F., Clark, J., Steele, S., ... Tager-Flusberg, H. (2004). Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage 22, 1141–1150. https://doi.org/10.1016/j.neuroimage.2004.03.025
Hall, G. B., Szechtman, H., & Nahmias, C. (2003). Enhanced salience and emotion recognition in autism: A PET study. American Journal of Psychiatry, 160, 1439–1441. https://doi.org/10.1176/appi.ajp.160.8.1439
Herrero, J. L., Khuvis, S., Yeagle, E., Cerf, M., & Mehta, A. D. (2018). Breathing above the brain stem: Volitional control and attentional modulation in humans. Journal of Neurophysiology, 119, 145–159. https://doi.org/10.1152/jn.00551.2017
Hietanen, J. O., Peltola, M. J., & Hietanen, J. K. (2020). Psychophysiological responses to eye contact in a live interaction and in video call. Psychophysiology, 57, e13587. https://doi.org/10.1111/psyp.13587
Hutt, C., Forrest, S. J., & Richer, J. (1975). Cardiac arrhythmia and behaviour in autistic children. Acta Psychiatrica Scand., 51, 361–372. https://doi.org/10.1111/j.1600-0447.1975.tb00014.x
Iacovella, V., & Hasson, U. (2011). The relationship between BOLD signal and autonomic nervous system functions: Implications for processing of “physiological noise.” Magnetic Resonance Imaging, 29, 1338–1345. https://doi.org/10.1016/j.mri.2011.03.006
Jarrold, C., & Brock, J. (2004). To match or not to match? Methodological issues in autism-related research. Journal of Autism and Developmental Disorders, 34, 81–86. https://doi.org/10.1023/B:JADD.0000018078.82542.ab
Kang, E., Keifer, C. M., Levy, E. J., Foss-Feig, J. H., McPartland, J. C., & Lerner, M. D. (2018). Atypicality of the N170 event-related potential in autism spectrum disorder: A meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 657–666. https://doi.org/10.1016/j.bpsc.2017.11.003
Karvonen, A., Kykyri, V. L., Kaartinen, J., Penttonen, M., & Seikkula, J. (2016). Sympathetic nervous system synchrony in couple therapy. Journal of Marital and Family Therapy, 42, 383–395. https://doi.org/10.1111/jmft.12152
Khalsa, S. S., Adolphs, R., Cameron, O. G., Critchley, H. D., Davenport, P. W., Feinstein, J. S., ... Zucker, N. (2018). Interoception and mental health: a roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3, 501–513. https://doi.org/10.1016/j.bpsc.2017.12.004
Kluger, D. S., & Gross, J. (2020). Depth and phase of respiration modulate cortico-muscular communication. NeuroImage, 222, 117272. https://doi.org/10.1016/j.neuroimage.2020.117272
Kluger, D. S., Balestrieri, E., Busch, N. A., & Gross, J. (2021). Respiration aligns perception with neural excitability. eLife, 10, e70907. https://doi.org/10.7554/eLife.70907
Krach, S., Kamp-Becker, I., Einhäuser, W., Sommer, J., Frässle, S., Jansen, A., Rademacher, L., Müller-Pinzler, L., Gazzola, V., & Paulus, F. M. (2015). Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism. Human Brain Mapping, 36, 4730–4744. https://doi.org/10.1002/hbm.22949
Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84, 394–421. https://doi.org/10.1016/j.biopsycho.2010.03.010
Kylliäinen, A., & Hietanen, J. K. (2006). Skin conductance responses to another person’s gaze in children with autism. Journal of Autism and Developmental Disorders, 36, 517–525. https://doi.org/10.1007/s10803-006-0091-4
Kylliäinen, A., Wallace, S., Coutanche, M. N., Leppänen, J. M., Cusack, J., Bailey, A. J., & Hietanen, J. K. (2012). Affective-motivational brain responses to direct gaze in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry, 53, 790–797. https://doi.org/10.1111/j.1469-7610.2011.02522.x
Lacey, B. C., & Lacey, J. I. (1978). Two-way communication between the heart and the brain: Significance of time within the cardiac cycle. American Psychologist, 33, 99. https://doi.org/10.1037/0003-066X.33.2.99
Lang, P.J., Bradley, M.M., Cuthbert, B.N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL.
Langley, J. N. (1903). The autonomic nervous system. Brain, 26, 1–26.
Leung, R. C., Pang, E. W., Anagnostou, E., & Taylor, M. J. (2018). Young adults with autism spectrum disorder show early atypical neural activity during emotional face processing. Frontiers in Human Neuroscience, 12, 57. https://doi.org/10.3389/fnhum.2018.00057
Loomes, R., Hull, L., & Mandy, W. P. L. (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 466–474. https://doi.org/10.1016/j.jaac.2017.03.013
Louwerse, A., Tulen, J. H. M., van der Geest, J. N., van der Ende, J., Verhulst, F. C., & Greaves-Lord, K. (2014). Autonomic responses to social pictures in ASD. Autism Research, 7, 17–27. https://doi.org/10.1002/aur.1327
Lydon, S., Healy, O., Reed, P., Mulhern, T., Hughes, B. M., & Goodwin, M. S. (2014). A systematic review of physiological reactivity to stimuli in autism. Developmental Neurorehabilitation, 19, 335–355. https://doi.org/10.3109/17518423.2014.971975
Mathôt, S. (2018). Pupillometry: Psychology, physiology, and function. Journal of Cognition, 1, 1–23. https://doi.org/10.5334/joc.18
McEwen, B. S. (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. https://doi.org/10.1111/j.1749-6632.1998.tb09546.x
McPartland, J., Dawson, G., Webb, S. J., Panagiotides, H., & Carver, L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 45, 1235–1245. https://doi.org/10.1111/j.1469-7610.2004.00318.x
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med., 6, e1000097. https://doi.org/10.1371/journal.pmed.1000097
O’Reilly, C., Lewis, J. D., & Elsabbagh, M. (2017). Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS One, 12, e0175870. https://doi.org/10.1371/journal.pone.0175870
Palkovitz, R. J., & Wiesenfeld, A. R. (1980). Differential autonomic responses of autistic and normal children. Journal of Autism and Developmental Disorders, 10, 347–360. https://doi.org/10.1007/BF02408294
Pardo, C. A., & Eberhart, C. G. (2007). The neurobiology of autism. Brain Pathology, 17, 434–447. https://doi.org/10.1111/j.1750-3639.2007.00102.x
Parviainen, T., Lyyra, P., Nokia, M. S. (2022) Cardiorespiratory rhythms, brain oscillatory activity and cognition: review of evidence and proposal for significance. Neuroscience & Biobehavioral Reviews. 104908. https://doi.org/10.1016/j.neubiorev.2022.104908.
Patriquin, M. A., DeRamus, T., Libero, L. E., Laird, A., & Kana, R. K. (2016). Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Human Brain Mapping, 37, 3957–3978. https://doi.org/10.1002/hbm.23288
Patriquin, M., Hartwig, E. M., Friedman, B. H., Porges, S. W., & Scarpa, A. (2019). Autonomic response in autism spectrum disorder: Relationship to social and cognitive functioning. Biological Psychology, 145, 185–197. https://doi.org/10.1016/j.biopsycho.2019.05.004
Pierce, K., Haist, F., Sedaghat, F., & Courchesne, E. (2004). The brain response to personally familiar faces in autism: Findings of fusiform activity and beyond. Brain, 127, 2703–2716. https://doi.org/10.1093/brain/awh289
Pönkänen, L. M., Alhoniemi, A., Leppänen, J. M., & Hietanen, J. K. (2011). Does it make a difference if I have an eye contact with you or with your picture? An ERP study. Social Cognitive and Affective Neuroscience, 6, 486–494. https://doi.org/10.1093/scan/nsq068
Port, R. G., Anwar, A. R., Ku, M., Carlson, G. C., Siegel, S. J., & Roberts, T. P. L. (2015). Prospective MEG biomarkers in ASD: pre-clinical evidence and clinical promise of electrophysiological signatures. The Yale Journal of Biology and Medicine., 88, 25–36.
Posada-Quintero, H. F., & Chon, K. H. (2020). Innovations in electrodermal activity data collection and signal processing: A systematic review. Sensors, 20, 479. https://doi.org/10.3390/s20020479
Quintana, D. S., Guastella, A. J., Outhred, T., Hickie, I. B., & Kemp, A. H. (2012). Heart rate variability is associated with emotion recognition: Direct evidence for a relationship between the autonomic nervous system and social cognition. International Journal of Psychophysiology, 86, 168–172. https://doi.org/10.1016/j.ijpsycho.2012.08.012
Redcay, E., Dodell-Feder, D., Mavros, P. L., Kleiner, M., Pearrow, M. J., Triantafyllou, C., Gabrieli, J. D., & Saxe, R. (2013). Atypical brain activation patterns during a face-to-face joint attention game in adults with autism spectrum disorder. Human Brain Mapping, 34, 2511–2523. https://doi.org/10.1002/hbm.22086
Richter, C. G., Babo-Rebelo, M., Schwartz, D., & Tallon-Baudry, C. (2017). Phase-amplitude coupling at the organism level: The amplitude of spontaneous alpha rhythm fluctuations varies with the phase of the infra-slow gastric basal rhythm. NeuroImage, 146, 951–958. https://doi.org/10.1016/j.neuroimage.2016.08.043
Schulte-Rüther, M., Greimel, E., Markowitsch, H. J., Kamp-Becker, I., Remschmidt, H., Fink, G. R., & Piefke, M. (2011). Dysfunctions in brain networks supporting empathy: An fMRI study in adults with autism spectrum disorders. Social Neuroscience, 6, 1–21. https://doi.org/10.1080/17470911003708032
Silvennoinen, M., Parviainen, T., Malinen, A., Karjalainen, S., Manu, M., Vesisenaho, M. (2022). Combining physiological and experiential measures to study the adult learning experience. In: Goller, M., Kyndt, E., Paloniemi, S., Damşa, C. (eds.) Methods for researching professional learning and development. Professional and practice-based learning, 33, 137–164. Springer, Cham. https://doi.org/10.1007/978-3-031-08518-5_7.
Smith, E. E., Reznik, S. J., Stewart, J. L., & Allen, J. J. B. (2017). Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. International Journal of Psychophysiology, 111, 98–114. https://doi.org/10.1016/j.ijpsycho.2016.11.005
Stanfield, A. C., McIntosh, A. M., Spencer, M. D., Philip, R., Gaur, S., & Lawrie, S. M. (2008). Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry, 23, 289–299. https://doi.org/10.1016/j.eurpsy.2007.05.006
Stevanovic, M., Henttonen, P., Koskinen, E., Peräkylä, A., Nieminen Von-Wendt, T., Sihvola, E., Tani, P., Ravaja, N., & Sams, M. (2019). Physiological responses to affiliation during conversation: comparing neurotypical males and males with Asperger syndrome. PLoS One, 14, e0222084. https://doi.org/10.1371/journal.pone.0222084
Tsakiris, M., & Critchley, H. (2016). Interoception beyond homeostasis: Affect, cognition and mental health. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 371, 20160002. https://doi.org/10.1098/rstb.2016.0002
Tseng, A., Wang, Z., Huo, Y., Goh, S., Russell, J. A., & Peterson, B. S. (2016). Differences in neural activity when processing emotional arousal and valence in autism spectrum disorders. Human Brain Mapping, 37, 443–461. https://doi.org/10.1002/hbm.23041
Van Overwalle, F. (2009). Social cognition and the brain: A meta-analysis. Human Brain Mapping, 30, 829–858. https://doi.org/10.1002/hbm.20547
Van Hecke, A. V., Lebow, J., Bal, E., Lamb, D., Harden, E., Kramer, A., Denver, J., Bazhenova, O., & Porges, S. W. (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development, 80, 1118–1133. https://doi.org/10.1111/j.1467-8624.2009.01320.x
von dem Hagen, E. A., Stoyanova, R. S., Rowe, J. B., Baron-Cohen, S., & Calder, A. J. (2014). Direct gaze elicits atypical activation of the theory-of-mind network in autism spectrum conditions. Cerebral Cortex, 24, 1485–1492. https://doi.org/10.1093/cercor/bht003
Wang, J., Barstein, J., Ethridge, L. E., Mosconi, M. W., Takarae, Y., & Sweeney, J. A. (2013). Resting state EEG abnormalities in autism spectrum disorders. Journal of Neurodevelopmental Disorders, 5, 24. https://doi.org/10.1186/1866-1955-5-24
Waselius, T., Wikgren, J., Halkola, H., Penttonen, M., & Nokia, M. S. (2018). Learning by heart: Cardiac cycle reveals an effective time window for learning. Journal of Neurophysiology, 120, 830–838. https://doi.org/10.1152/jn.00128.2018
Waselius, T., Wikgren, J., Penttonen, M., & Nokia, M. S. (2019). Breathe out and learn: expiration-contingent stimulus presentation facilitates associative learning in trace eyeblink conditioning. Psychophysiology, 56, e13387. https://doi.org/10.1111/psyp.13387
Funding
Open Access funding provided by University of Jyväskylä (JYU). This work was supported by the Academy of Finland [grant number 311877], the Finnish Cultural Foundation and the Central Finland Regional Fund of the Finnish Cultural Foundation.
Author information
Authors and Affiliations
Contributions
SK: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft.
TA: Conceptualization, Validation, Writing – Review & Editing, Supervision.
TP: Conceptualization, Formal analysis, Investigation, Validation, Writing – Review & Editing, Supervision.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karjalainen, S., Aro, T. & Parviainen, T. Coactivation of Autonomic and Central Nervous Systems During Processing of Socially Relevant Information in Autism Spectrum Disorder: A Systematic Review. Neuropsychol Rev 34, 214–231 (2024). https://doi.org/10.1007/s11065-023-09579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-023-09579-2