Costs of Persons with Dementia Living in Nursing Homes in The Netherlands
Abstract
Background:
Disease modifying treatments (DMTs) currently under development for Alzheimer’s disease, have the potential to prevent or postpone institutionalization and more expensive care and might delay institutionalization of persons with dementia.
Objective:
The current study estimates costs of living in a nursing home for persons with dementia in the Netherlands to help inform economic evaluations of future DMTs.
Methods:
Data were collected during semi-structured interviews with healthcare professionals and from the financial administration of a healthcare organization with several nursing homes. Personnel costs were calculated using a bottom-up approach by valuing the time estimates. Non-personnel costs were calculated using information from the financial administration of the healthcare organization.
Results:
Total costs of a person with dementia per 24 hours, including both care staff and other healthcare providers, were € 151 for small-scale living wards and € 147 for independent living wards. Non-personnel costs were € 37 per day.
Conclusion:
This study provides Dutch estimates for total healthcare costs per day for institutionalized persons with dementia. These cost estimates can be used in cost-effectiveness analyses for future DMTs in dementia.
INTRODUCTION
Dementia is an umbrella term for a group of symptoms associated with an ongoing decline in brain functioning (i.e., impaired memory, language, problem-solving, or other thinking abilities) severe enough to affect a patient’s daily life. Approximately 55 million people are diagnosed with dementia worldwide [1]. Due to aging, the prevalence of dementia is expected to increase to 78 million in 2030 and 139 million in 2050 [1]. The global incidence of dementia is calculated at 10 million patients annually. Dementia ranked fifth in leading causes of death worldwide in 2016 [2]. With a share of about 60 to 70%, Alzheimer’s disease is the most common cause of dementia [1].
With disease progression, persons with dementia increasingly require care and assistance with daily activities. Eventually, many persons with dementia are in need of care or supervision 24 hours a day, at which point they are often admitted to a nursing home. Consequently, health systems are challenged to deal with the predicted future increase of prevalence of dementia, with important implications for both staffing and financial resources.
In 2019, the global cost of dementia was estimated at $1.3 trillion and are projected to reach $2.8 trillion by 2030, as both the prevalence of dementia and healthcare costs are expected to increase globally [1]. The majority of costs associated with dementia care are related to direct social care costs (including institutionalization) [3]. Disease modifying treatments (DMTs) currently under development for Alzheimer’s disease, have the potential to prevent or postpone institutionalization and more expensive care [4–8]. Part of the acquisition costs of DMT may be offset by savings from preventing or postponing institutionalization of persons with dementia. This and other economic aspects of DMTs can be included in costs-effectiveness analyses, alongside effects of DMTs. Cost-effectiveness analyses can inform decision makers on optimal budget allocation, providing insight into whether health benefits outweigh the cost consequences of an intervention. In order to have an accurate estimate of potential economic impact of DMTs, including potential savings with regard to institutionalization, knowledge on costs of institutionalization is essential. There have been various studies on healthcare utilization and costs incurred by persons with dementia in the European region [9–12]. However, unit costs of healthcare are considered to exhibit low transferability [13]. In the Netherlands, cost prices have been calculated for care profiles (i.e., broad categories of intensity of care provided in institutions) [14]. However, these care profiles are not condition-specific, and cost prices for care for dementia are not available. Wübker et al. (2015) also presented costs for nursing home stays in the Netherlands; however, the cost inputs used in this study were not specific for persons with dementia [15]. Therefore, the objective of the current study was to estimate the costs of living in a nursing home for a person with dementia in the Netherlands to feed Dutch pharmacoeconomic models for DMTs for Alzheimer’s disease.
MATERIALS AND METHODS
Data collection
Data on the time healthcare providers spend on care for dementia were collected during semi-structured interviews with healthcare professionals and from the financial administration of a healthcare organization with several nursing homes. This organization offers district nursing, rehabilitation care, and long-term care (no hospital care) to approximately 2000 patients at 16 locations of different sizes in a specific region in the Netherlands. As is commonly seen in the Netherlands, the nursing homes included in the study have two types of wards where persons with dementia can reside: small-scale psychogeriatric living groups (in Dutch: ‘kleinschalig wonen’ or ‘psychogeriatrische afdeling’) and independent living (in Dutch: ‘open afdeling’), both with 24-hour healthcare provision. In small-scale living wards, 6 or 7 residents form a household with a shared living room and kitchen but with private bedrooms and bathrooms. In independent living wards, residents have their own private apartment, but do have access to 24-hour healthcare and activities.
Interviews
Interviews were performed with at least two researchers (PK, SH, VW, TK) during a video call (physical presence was undesired due to the COVID-19 pandemic regulations) with an average duration of 60–75 minutes without any financial compensation for respondents. Interviews took place in April and May 2021. Respondents were recruited through a central inquiry in the organization. All respondents participated on a voluntary basis.
Respondents consisted of care staff, a physician (geriatrist), therapists, and an activity supervisor. In the Netherlands, care staff in nursing homes can be divided into three job groups: assistant, caregiver, and nurse. In addition to care staff, persons with dementia receive healthcare from a physician (specialist in geriatric medicine) and several therapists (i.e., an occupational therapist, a physiotherapist, a psychologist, and a nurse with special attention for diets). Finally, activity supervisors organize individual and group activities for residents in the nursing homes.
The interview template varied for care staff and other healthcare professionals. Interviews with care staff started with general questions about the respondent’s job, experience with caring for persons with dementia, and the number of persons with dementia at their ward. The main goal of the interviews was to collect information about the time healthcare providers spend on the care for dementia. To extract this information, a table with time spent per task and shifts (i.e., day, evening, and night) was completed during the interviews. Tasks included personal care, social care, medical care, administration, updates about residents (i.e., reading patients dossiers as preparation before shift and consultation with colleagues about residents), and other tasks. Finally, open-ended questions were asked about workload, and the impact of COVID-19 on healthcare provision in nursing homes. These open-ended questions were included to be able to narratively describe the impact of these topics on the daily work of the care staff and other healthcare professionals. Interviews with the other healthcare professionals followed the same structure; however, the breakdown of time spending was not deemed relevant for this group of respondents, as they had different tasks and did not have daily contact with every resident. Therefore, the average weekly frequency and duration of contact were collected. The interview template is provided in the Supplementary Material.
Financial administrative data of the healthcare organization
Total costs and number of annual patients of the healthcare organization in 2019 were retrieved from their financial administration and included the following cost categories: personnel, depreciation, food and hotel, overhead, patient and resident, maintenance and energy, rent and leasing, and contribution and release of provisions. Total number of annual patients of the healthcare organization were divided into patients receiving intramural (i.e., patients residing in a healthcare institution) and extramural care (i.e., patients not residing in an institution).
The distributions of care staff functions (i.e., assistants, caregivers, and nurses) in the nursing home in small-scale living wards and independent living wards, not specific for dementia care, were based on full-time equivalent (FTE) employment data retrieved from the financial administration.
Phases of dementia
Dementia can be categorized in four severity classes: mild cognitive impairment (MCI), mild dementia, moderate dementia, and severe dementia. To refrain from using clinical dementia rating unknown to respondents, descriptions of phases of dementia (Supplementary Material) were constructed to guide the interviews and enable respondents to identify differences between severity. According to the interviewed geriatrist, persons admitted to a nursing home primarily for dementia typically have moderate or severe dementia. In the Netherlands, reimbursement of nursing homes by healthcare insurers is based on care profiles (in Dutch: ‘zorgprofielen’ or formerly ‘zorgzwaartepakketten [ZZP]’). There are three care profiles for persons with dementia living in nursing homes: ‘ZZP 4: Sheltered living with intensive supervision and extensive care’, ‘ZZP 5: Protected living with intensive dementia care’, and ‘ZZP 7: Protected living with very intensive care, due to specific disorders, with an emphasis on guidance’. The distribution of patients across these care profiles were retrieved from the financial administrative data of the healthcare organization, separately for small-scale living wards and independent living wards.
Personnel costs
Personnel costs were calculated using a bottom-up approach by valuing the time estimates collected in the interviews with healthcare professionals.
Interview data was used to calculate the average time spent on every type of task per shift and the average total time spent on persons with dementia by care staff in nursing homes. Time estimations of all care staff members were combined (i.e., assistants, caregivers, and nurses) because of the similarity of type of tasks they perform. For example, assistants can take over tasks from caregivers and nurses. In addition, persons in nursing homes do not require much medical care.
Interviews with other healthcare professionals (i.e., physician, therapists, and activity supervisor) were performed to estimate the average time spent per week on persons with dementia for each type of healthcare provider. To guarantee anonymity of respondents, individual time estimates per type of healthcare provider were not reported.
Time estimates from the interviews were multiplied with healthcare providers’ hourly wages to estimate personnel costs. Yearly pensionable salaries were provided by the financial administration. Irregularity allowances, holiday allowance and year-end bonus were included in the pensionable salaries. Social security costs and other personnel costs were taken from the Dutch costing manual [16]. The distribution of hours over the different types of care staff members was based on FTE data from the nursing home for day and evening shifts. Staff present during night shifts was assumed to consist exclusively of caregivers or nurses (i.e., no assistants). An FTE was assumed to have 1536 workable hours per year [16].
Other costs
Non-personnel costs were calculated using information from the financial administration of the healthcare organization. The data did not distinguish between intramural and extramural non-personnel costs. General costs were assumed to be distributed evenly across intramural and extramural patients. Food and hotel costs were only assigned to patients receiving intramural care. Other non-personnel costs (i.e., depreciation, patient and resident related costs, maintenance and energy costs, rent and leasing, and contribution and release of provisions), were corrected for the difference in time that intramural and extramural patients use the care and facilities of the healthcare organization by assuming that intramural patients receive twice as much care time compared to extramural patients. Subsequently, these costs were divided by the number of intramural patients in the healthcare organization and divided by 365.25 to calculate the costs per patient per day.
RESULTS
Interview respondents
In total, 19 healthcare staff members in the nursing home were interviewed: 7 care staff members working at small-scale living wards (1 assistant, 3 caregivers, and 3 nurses), 3 care staff members working at independent living wards (1 caregiver and 2 nurses), one specialist in geriatric medicine, one occupational therapist, two physiotherapists, two psychologists, a directing nurse, a nurse with special attention for diets, and one activity supervisor.
Care staff teams working at small-scale living wards were responsible for 6 to 7 residents during the day and evening and around 25 residents during the night (i.e., four small-scale living wards), while the care staff teams working at independent living wards and activity supervisor were responsible for 14–22 residents. The physician and therapists were responsible for 60 to 150 patients, but only a proportion of their patients required treatment of the physician and therapists.
Phases of dementia and care profiles
According to respondents, the majority of residents in small-scale living wards (94%) had some type of dementia, 48% with moderate dementia and 52% with severe dementia. All residents without dementia in small-scale living wards had Parkinson’s disease, except for one patient with somatic complaints. In the independent living wards, 54% of the residents had dementia: 95% moderate dementia and 5% severe dementia. The remaining 46% were primarily living in nursing homes because of somatic complaints.
Cost calculations
Personnel costs. Tables 1 and 2 present the average number of minutes per day care staff in nursing homes spend per person with dementia in small-scale living wards and independent living wards. Respondents working in independent living wards did not provide estimates for time spent during night shifts. Therefore, the estimate from the small-scale living ward was used as a proxy. According to respondents, time spent on care tasks did not differ for persons with moderate or severe dementia. Therefore, the estimates represent an average for both moderate and severe dementia.
Table 1
Average time spent by care staff on persons with dementia in small-scale living wards
| Shift | Day | Evening | Night | Total (24 h) |
| Number of interviewed care staff members | 7 | 7 | 7 | |
| Tasks (minutes per person with dementia) | ||||
| Personal care | 49 | 27 | 9 | 85 |
| Social care | 26 | 9 | 4 | 39 |
| Medical care | 8 | 7 | 0 | 15 |
| Administration | 8 | 5 | 2 | 15 |
| Update about residents* | 10 | 4 | 2 | 16 |
| Other tasks | 39 | 13 | 2 | 55 |
| Minutes per person with dementia | 124 | 81 | 15 | 220 |
| Distribution of care staff** | ||||
| Assistants | 28.9% | 28.9% | ||
| Caregivers | 60.7% | 60.7% | 85.4% | |
| Nurses | 10.4% | 10.4% | 14.6% | |
| Costs per person with dementia | € 57.31 | € 37.62 | € 7.15 | € 102.08 |
*Reading patients dossiers as preparation before shift and consultation with colleagues about residents. **Distribution was based on administrative data provided by the healthcare organization.
Table 2
Average time spent by care staff on persons with dementia in independent living wards
| Shift | Day | Evening | Night*** | Total (24 h) |
| Number of interviewed care staff members | 3 | 2 | 0 | |
| Tasks (minutes per person with dementia) | ||||
| Personal care | 33 | 19 | 52 | |
| Social care | 22 | 25 | 47 | |
| Medical care | 22 | 10 | 32 | |
| Administration | 9 | 18 | 27 | |
| Update about residents* | 8 | 18 | 26 | |
| Other tasks | 5 | 8 | 13 | |
| Minutes per person with dementia | 99 | 98 | 15 | 213 |
| Distribution of care staff** | ||||
| Assistants | 28.9% | 28.9% | ||
| Caregivers | 60.7% | 60.7% | 85.4% | |
| Nurses | 10.4% | 10.4% | 14.6% | |
| Costs per person with dementia | € 45.95 | € 45.31 | € 7.22 | € 98.48 |
*Reading patient records as preparation before shift and consultation with colleagues about residents. **Distribution was based on administrative data provided by the healthcare organization. ***There are night shifts in independent living wards, as no care staff members who worked night shifts were interviewed, the estimate from the small-scale living wards was used (15 minutes per night).
Most time of care staff was spent on personal care tasks, such as personal hygiene and helping persons with dementia getting in and out of bed. Social care was often provided while performing other tasks such as personal care and not counted as social care time to make both mutually exclusive. Therefore, time estimates reported here for social care tasks underestimate the total time spent on it. In total, care staff spend 220 minutes (=3.7 hours; SD: 25.3) and 213 minutes (=3.6 hours; SD: 68.2) per 24 hours per person with dementia in small-scale living wards and independent living wards, respectively. This was associated with personnel costs of € 102.08 per 24 hours per person with dementia in small-scale living wards and € 98.48 per 24 hours in independent living wards.
On average, each of the other healthcare professionals (paramedics and other non-direct caregiving staff) spent 19 minutes (range 11–34 minutes) per week per person with dementia. This was associated with a cost of € 12.45 per day per person with dementia (Table 3). Time estimates of other healthcare professionals were not distinguished between small-scale living and independent living wards.
Table 3
Total cost of institutionalization per client per 24 hours
| Type of cost | Small-scale living | Independent living |
| Personnel costs caregiving staff | € 102.08 | € 98.48 |
| Other healthcare professionals | € 12.45 | € 12.45 |
| Other costs | € 36.53 | € 36.53 |
| Total costs | € 151.06 | € 147.46 |
Other costs and total costs. Other costs consisted of food and hotel costs, overhead, maintenance and energy, rent and lease and other costs. These costs amounted to € 37 per 24 hours. Total costs estimates are presented in Table 3. Total cost per person with dementia was € 151 per 24 hours for small-scale living facilities and € 147 for independent living facilities. In the healthcare organization, 55.1% of persons were living in small-scale living facilities. The weighted average total cost per person with dementia was € 149 per 24 hours.
Caregiver workload and impact of COVID-19
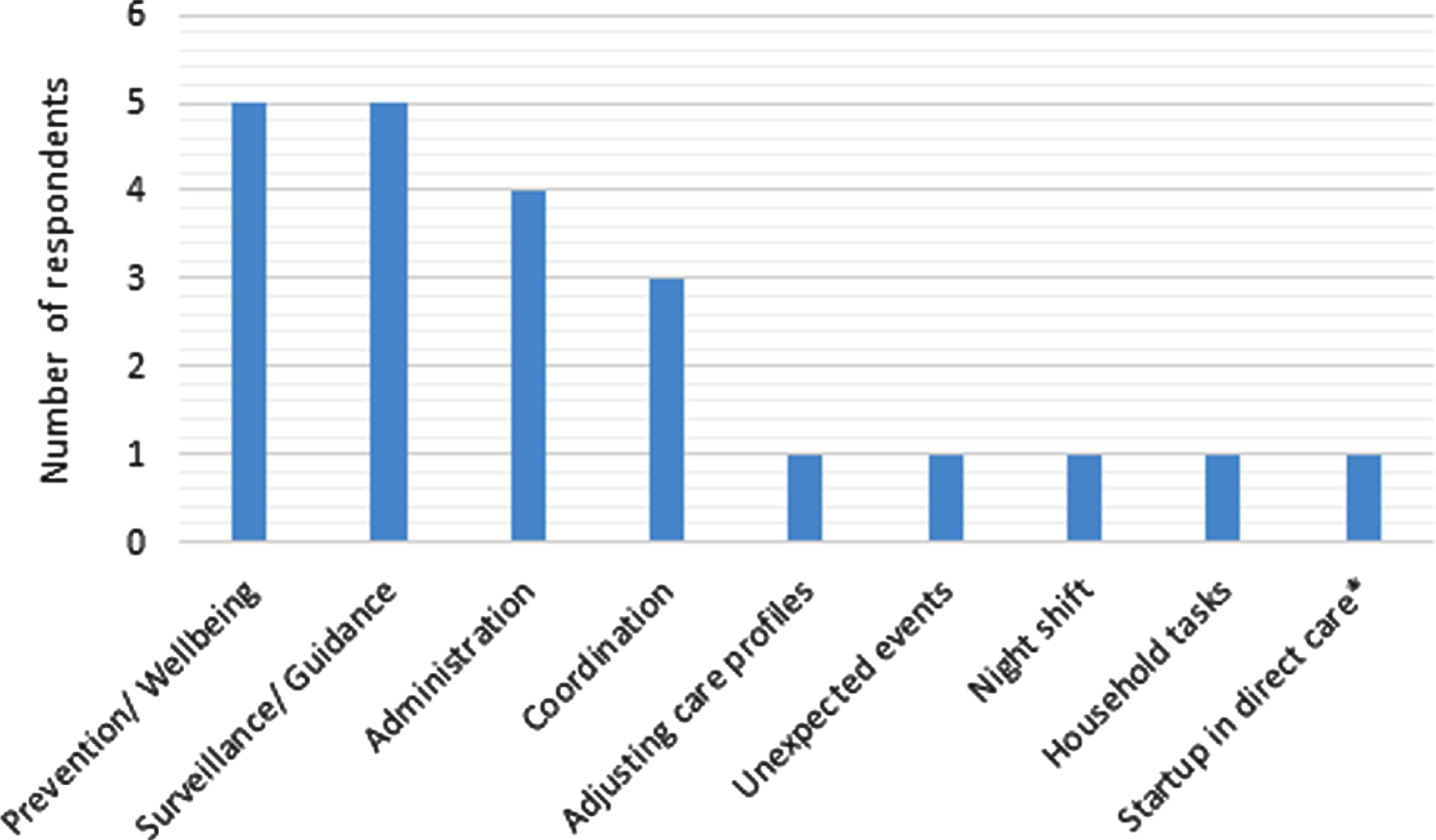
Most respondents (84%) indicated that there are (care) tasks that cannot currently be performed properly within the budgeted time. Only three respondents (nurses of varying function levels; working at different locations) were able to provide all the care that persons with dementia need. Figure 1 provides an overview of the activities respondents were unable to perform properly.
Fig. 1
Activities respondents are unable to carryout due to budget and/or time constraints. Prevention/Wellbeing activities were mostly aimed at mobility and included for example cycling and walking. Surveillance/Guidance activities include observing behavior and helping patients cope. Coordination was defined as coordinating tasks between different caregivers. Night shift was mainly discussed in terms of being generally understaffed. *respondent’s job profile is not fully focused on direct caregiving and involves management related tasks.
The majority of respondents (14; 74%) indicated that they had worked more hours due to COVID-19. In contrast, 4 (21%) respondents indicated that they worked less during the peak of the COVID-19 pandemic. The pandemic caused a shift for some staff concerning their daily (care) tasks. For example, some caregivers devoted themselves completely to the planning of shifts and thus no longer performed any caregiving tasks during the height of the pandemic. Furthermore, the focus of care more often shifted to counseling and well-being of persons with dementia. In this case, well-being relates to tasks and activities which improve the health and happiness of persons with dementia outside of direct care tasks (e.g., going for a walk, cycling, etc.). The (daytime) activities were largely canceled causing the patients to be on the wards all day. To prevent loneliness and inactivity, one-on-one activities were undertaken, which required more attention and time from staff than joint activities.
The majority of respondents indicated that the workload (at the peak of the COVID-19 pandemic) was very high, due to substantial degree of COVID-19 and stress related illness and absenteeism among staff members. In addition, COVID-19-related deaths amongst patients placed an emotional burden on staff.
Family and acquaintances were not, or only rarely allowed to visit in periods of lockdown during the pandemic. Residents often did not understand why family did not visit, which caused increased levels of loneliness. In addition, almost all terminal care and well-being (e.g., holding hands and sitting at the bedside) was performed by care assistants. Oppositely, one respondent commented that reduced family visits also allowed more peace of mind for caregivers to care for residents.
Most of the caregivers stated that their caregiving tasks had (almost) returned to pre-COVID-19 levels during the time of the interviews.
DISCUSSION
This study estimated the total costs of a person with dementia per day in a nursing home including both care staff and other healthcare providers in service of the nursing home. The total cost per day was estimated to be € 151 for small-scale living wards and € 147 for independent living wards.
In 2018, cost prices were calculated for long-term care for all care profiles in the Netherlands [14]. When applying the care profile distribution of the participating nursing home to these cost prices, the cost per person per day was € 159. The current cost estimate (weighted average € 149) is comparable to the KMPG estimate. To date, (time-and-motion) studies in the international literature have taken the perspective of the time spending of nurses or caregivers for multiple residents. This study investigated the cost of institutionalization per person with dementia, which prohibits comparisons of results to the international literature.
The majority of caregivers indicated that they did not have enough time to perform all required caregiving tasks properly, particularly, tasks related to prevention and/or wellbeing of persons with dementia. This contrasts with the Dutch Ministry of Health’s aim to emphasize on prevention in healthcare [17]. An upcoming Dutch law should enhance purchasing parties to invest in preventive measures to prevent or postpone more expensive care. Excessive workload has previously been shown to have a negative effect on quality of care [18] and on caregivers’ own health [19]. Increasing numbers of residents will likely only increase the workload of caregivers which is detrimental to the quality (and safety of) care provided [20], emphasizing the need for adequate policies to support caregiving staff and prevent understaffing.
Limitations
Information was obtained using online interviews, rather than on-site time and motion data collection. On-site time and motion studies can provide a greater level of detail and is not prone to potential recall bias of respondents. Time and motion studies would also enable cost variations according to degree of cognitive function and comorbidities. However, due to the COVID-19 pandemic and related restrictions, care facilities were reluctant to allow external researchers to enter the facilities out of concern for the safety of the residents and caregivers.
This study was conducted in a single long-term healthcare organization. During the COVID-19 pandemic, it proved difficult to include additional organizations. Nonetheless, the participating organization is rather large with 16 locations (rural and urban) with different functionalities (including small-scale living and independent living). In addition, the cost price estimated in this study was comparable to the KPMG estimate. These two elements suggest that the results are likely generalizable to Dutch nursing homes as a whole. Future studies in other organizations are needed to confirm the generalizability. Furthermore, although personnel with different job profiles were interviewed, the sample was relatively small, especially for independent living wards.
Data on time during night shifts was not collected in independent living wards. Still, respondents mentioned that night shifts are typically performed by one caregiver responsible for multiple wards, whereas during the day multiple caregivers are present in every single ward. Therefore, the costs associated with the night shift on the independent living wards are not expected to have a major impact on the total costs per day. In addition, the data did not allow to differentiate food costs between intra- and extramural patients. Therefore, it was assumed that extramural patients did not incur food costs. Overall, this assumption had a minor impact on total costs per day.
For this study, it was not possible to adequately differentiate between Alzheimer’s disease and other types of dementia. According to respondents, a diagnosis of dementia is in most cases in the Netherlands not further specified by type of dementia. Even though different causes of dementia can lead to a different clinical presentation, respondents experienced difficulties to categorize persons with dementia according to type of dementia. Nonetheless, most respondents declared that, except for vascular dementia in which persons typically exhibit more behavioral symptoms such as aggression and agitation, care needs of institutionalized. Persons with Alzheimer’s disease do not substantially differ from persons with other causes of dementia.
In conclusion, this study provides Dutch estimates for total healthcare costs per day for institutionalized persons with dementia. These cost estimates can potentially be used in cost-effectiveness analyses for future DMTs in dementia and specifically for Alzheimer’s disease.
ACKNOWLEDGMENTS
This paper and the research behind it would not have been possible without the help and openness of a Dutch healthcare organization, which will remain unnamed. This organization assisted us in scheduling interviews with its caregivers, and furthermore provided data from its financial administration for the purpose of this study without remuneration.
This work was sponsored by Biogen Netherlands B.V. through a contracted study grant. iMTA conducted this study independently. The funding party had no role in design, data collection and analysis of the study, nor did they have a role in the decision to publish or the preparation of the manuscript.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0416r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-220416.
REFERENCES
[1] | World Health Organization (2021) Factsheet Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia, Accessed on November 18, 2021). |
[2] | GBD 2016 Dementia Collaborators ((2019) ) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 88–106. |
[3] | Wimo A , Guerchet M , Ali G-C , Wu Y-T , Prina AM , Winblad B , Jönsson L , Liu Z , Prince M ((2017) ) The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13: , 1–7>. |
[4] | Green C , Handels R , Gustavsson A , Wimo A , Winblad B , Sköldunger A , Jönsson L ((2019) ) Assessing cost-effectiveness of early intervention in Alzheimer’s disease: An open-source modeling framework. Alzheimers Dement 15: , 1309–1321. |
[5] | Wimo A , Handels R , Winblad B , Black CM , Johansson G , Salomonsson S , Eriksdotter M , Khandker RK ((2020) ) Quantifying and describing the natural history and costs of Alzheimer’s disease and effects of hypothetical interventions. J Alzheimers Dis 75: , 891–902. |
[6] | Budd D , Burns LC , Guo Z , L’italien G , Lapuerta P ((2011) ) Impact of early intervention and disease modification in patients with predementia Alzheimer’s disease: A Markov model simulation. Clinicoecon Outcomes Res 3: , 189–195. |
[7] | Anderson R , Knapp M , Wittenberg R , Handels R , Schott JM (2018) Economic modelling of disease-modifying therapies in Alzheimer–s disease, Personal Social Services Research Unit, London. |
[8] | Sköldunger A , Johnell K , Winblad B , Wimo A ((2013) ) Mortality and treatment costs have a great impact on the cost-effectiveness of disease modifying treatment in Alzheimer’s disease–a simulation study. Curr Alzheimer Res 10: , 207–216. |
[9] | Luengo-Fernandez R , Leal J , Gray AM ((2011) ) Cost of dementia in the pre-enlargement countries of the European Union. J Alzheimers Dis 27: , 187–196. |
[10] | Wimo A , Jönsson L , Gustavsson A , McDaid D , Ersek K , Georges J , Gulácsi L , Karpati K , Kenigsberg P , Valtonen H ((2011) ) The economic impact of dementia in Europe in 2008— cost estimates from the Eurocode project. Int J Geriatr Psychiatry 26: , 825–832. |
[11] | Jönsson L , Wimo A ((2009) ) The cost of dementia in Europe. Pharmacoeconomics 27: , 391–403. |
[12] | Schaller S , Mauskopf J , Kriza C , Wahlster P , Kolominsky-Rabas PL ((2015) ) The main cost drivers in dementia: A systematic review. Int J Geriatr Psychiatry 30: , 111–129. |
[13] | Barbieri M , Drummond M , Rutten F , Cook J , Glick HA , Lis J , Reed SD , Sculpher M , Severens JL ((2010) ) What do international pharmacoeconomic guidelines say about economic data transferability? . Value Health 13: , 1028–1037. |
[14] | KPMG (2018) KPMG Costing study longterm care [in Dutch: KPMG Kostenonderzoek langdurige zorg]. Nederlandse Zorgautoriteit, Utrecht. |
[15] | Wübker A , Zwakhalen SMG , Challis D , Suhonen R , Karlsson S , Zabalegui A , Soto M , Saks K , Sauerland ((2015) ) Costs of care for people with dementia just before and after nursing home placement: Primary data from eight European countries. Eur J Health Econ 16: , 689–707. |
[16] | Hakkaart-van Rooijen L , Van der Linden N , Bouwmans C , Kanters T , Tan SS (2015) Costing manual: Methodology of costing research and reference prices for economic evaluations in healthcare [in Dutch: Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg], Zorginstituut Nederland, Diemen. |
[17] | Tweede Kamer der Staten-Generaal (2016) Prevention healthcare policy [in Dutch: Preventief gezondheidsbeleid], ’ Tweede Kamer der Staten-Generaal, s-Gravenhage. |
[18] | Buljac-Samardzić M , Van Woerkom M ((2018) ) Improving quality and safety of care in nursing homes by team support for strengths use: A survey study. PLoS One 13: , e0200065. |
[19] | Kandelman N , Mazars T , Levy A ((2018) ) Risk factors for burnout among caregivers working in nursing homes. J Clin Nurs 27: , e147–e153. |
[20] | Peck Malliaris A , Phillips J , Bakerjian D , Nursing and Patient Safety. https://psnet.ahrq.gov/primer/nursing-and-patient-safety, Last updated April 21, 2021, Accessed on December 15, 2021. |



