Right-touch regulation
Catherine Reilly speaks to outgoing Medical Council President Dr Rita Doyle about an eventful three years in office

Public/private mix
Paul Mulholland examines recent HSE documents regarding its monitoring of the private activity carried out by consultants

PAGE 10
FMT during Covid-19
Faecal microbiota transplant (FMT) services internationally were severely impacted by Covid-19. David Lynch explores the effect of the pandemic on FMT in Ireland
PAGE 14
Thwarting the next health catastrophe
Dr Muiris Houston asks if an empowered World Health Organisation would help prevent future pandemics
PAGE 16
No central collation of Covid-19 staff derogations – HSE
CATHERINE REILLY
There is “no central collation” of data on Covid-19 staff derogations during the pandemic in either acute or community sites, according to the HSE.
“This was a local issue and was managed by local managers,” a spokesperson informed the Medical Independent (MI).
The HSE’s derogations policy permits healthcare workers to be brought back to work while restricting their movements (eg, as a close contact) if certain criteria are met. The healthcare worker must be “essential to critical service needs”.
However, healthcare unions have said the policy was misused.
In October 2020, Director of Industrial Relations at the Irish Nurses and Midwives Organisation, Mr Tony Fitzpatrick, told MI local managers had been “derogating left, right, and centre” due to staffing deficits.
At the time, Dr Lynda Sisson, Clinical Lead of the HSE Workplace Health and Wellbeing Unit, told MI the data was not collected and examined at national level, but efforts were being made to address this.
According to an email obtained under Freedom of Information law, on 11 January 2021 Dr Sisson requested National Director of Community Operations Ms Yvonne O’Neill to “confirm that there is a process in place to monitor the number and type of derogations in the community”.
Ms O’Neill responded that she would “ensure that there is a reporting process put in place for cases of derogation”.
The derogations policy was most recently revised on 12 March 2021. It specified derogation cannot be applied to healthcare workers “who are self-isolating following travel from a country with a Covid-19 ‘variant of concern’” or identified as close
contacts of confirmed/suspected cases of a variant of concern.
In terms of being a close contact of a suspected or confirmed Covid-19 case in their home (household contacts), healthcare workers can only be derogated in “very exceptional circumstances”.
Approval for these derogations must be received from the Office of the National Director of Acute Operations or the Office of the National Director of Community Operations, outlined the policy.
All derogated healthcare workers must have a negative test immediately prior to returning to the workplace, according to the policy.

In regard to vaccination, the policy stated: “Healthcare workers who are close contacts and have completed the full Covid-19 vaccination course and the vaccine-specific time period to achieve full immunity (as per the licensed indications) can be considered for derogation from restricted
movements, in preference over other healthcare workers.”
“Currently, this is limited to those vaccinated within the two






Sláintecare contract concerns
DAVID LYNCH
The IHCA and IMO have raised significant
“The current approach to addressing the significant consultant shortages in our system lacks ambition and strategic thinking.
months from when vaccine immunity is reached, given the current maximum follow-up data for the licensed vaccines.”
Vimovo® is indicated in adults for the symptomatic treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis, in patients who are at risk for developing non-steroidal anti-inflammatory drug (NSAID) -associated gastric and/or duodenal ulcers and where treatment with lower doses of naproxen or of other NSAIDs is not considered sufficient.1

On the initial meeting the spokesperson said: “Alarmingly, we were informed by the officials that they had ‘no mandate’ to engage in discussions on the very matters that were, outwardly at least, the purpose of the meeting.”
“It is unclear how the particular proposals fit with the pressing need and strategy aimed at bucking the upward trends in growing hospital waiting lists and waiting times, unfilled consultant posts, and the departure of consultants to other health systems abroad.”
The IMO consultant committee was also unhappy with its meeting.
“The position of the Department is that the matters relating to this contract are not for negotiation, but for implementation with a provision for ‘feedback’,” according to an email sent to consultant members.
The IMO has advised the Department of its entitlement to negotiate contractual terms and conditions.
OUR PAPER IS NOW COMPOSTABLE, AS WELL AS RECYCLABLE NEWS 1-14 ● OPINION 15-16 ● CLINICAL 19-32 ● LIFE QUIZZES 34 ● FOOD & DRINK 35 ● GALLERY 36 ● RXDX 37 ● RECRUITMENT 38 31 MAY 2021 ● ISSUE 13 VOLUME 12 ● NEXT ISSUE 10 JUNE 2021 €5.95 PAGE 4-5
Reference: 1. Vimovo Summary of Product Characteristics. Legal classification: POM. Marketing Authorisation number, pack sizes: PA 1019/024/001, 60 packs. Marketing Authorisation Holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Further information is available upon request from Grünenthal Pharma Ltd, 4045 Kingswood Road, Citywest Business Park, Citywest, Co Dublin. M-VMO-IE-10-20-0002 – October 2020
Dr Connie Dale celebrates receiving her final year results from the RCSI with her one-month-old son Odhran and son Otto (aged one-and-a-half) at home in Dundrum, Dublin. More than 300 future doctors received their results on Thursday 13 May, marking the second time the RCSI results day event was held via live stream.
Picture credit: Julien Behal Photography
5 ADDITIONAL INDICATIONS NOW PUBLICLY AVAILABLE* IN IRELAND
KEYTRUDA, now publicly available for 5 additional indications, bringing the total number of indications now publicly available in Ireland to 9.
NSCLC
• NEW In combination with pemetrexed and platinum chemotherapy for the firstline treatment of metastatic non-squamous non-small cell lung carcinoma in adults whose tumours have no EGFR or ALK positive mutations.
UROTHELIAL CARCINOMA
• NEW As monotherapy for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy.
MELANOMA

KEYTRUDA® (pembrolizumab)

• NEW In combination with carboplatin and paclitaxel for the first-line treatment of patients with metastatic squamous non-small cell lung cancer (NSCLC).
• NEW As monotherapy for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD-L1 with a combined positive score (CPS) ≥10.
• NEW As monotherapy is indicated for the adjuvant treatment of adults with Stage III melanoma and lymph node involvement who have undergone complete resection.

ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing. PRESENTATION KEYTRUDA 25 mg/mL: One vial of 4 mL of concentrate contains 100 mg of pembrolizumab. INDICATIONS KEYTRUDA as monotherapy is indicated for the treatment of advanced (unresectable or metastatic) melanoma in adults. KEYTRUDA as monotherapy is indicated for the adjuvant treatment of adults with Stage III melanoma and lymph node involvement who have undergone complete resection. KEYTRUDA as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumours express PD-L1 with a ≥50% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations. KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic non-squamous NSCLC in adults whose tumours have no EGFR or ALK positive mutations. KEYTRUDA, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous NSCLC in adults. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic NSCLC in adults whose tumours express PD-L1 with a ≥1% TPS and who have received at least one prior chemotherapy regimen. Patients with EGFR or ALK positive tumour mutations should also have received targeted therapy before receiving KEYTRUDA. KEYTRUDA as monotherapy is indicated for the treatment of adult and paediatric patients aged 3 years and older with relapsed or refractory classical Hodgkin lymphoma (cHL) who have failed autologous stem cell transplant (ASCT) or following at least two prior therapies when ASCT is not a treatment option. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who have received prior platinum-containing chemotherapy. KEYTRUDA as monotherapy is indicated for the treatment of locally advanced or metastatic urothelial carcinoma in adults who are not eligible for cisplatin-containing chemotherapy and whose tumours express PD L1 with a combined positive score (CPS) ≥ 10. KEYTRUDA as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy, is indicated for the first-line treatment of metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC) in adults whose tumours express PD-L1 with a CPS ≥ 1. KEYTRUDA as monotherapy is indicated for the treatment of recurrent or metastatic HNSCC in adults whose tumours express PD-L1 with a ≥ 50% TPS and progressing on or after platinum-containing chemotherapy. KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of advanced renal cell carcinoma (RCC) in adults. KEYTRUDA as monotherapy is indicated for the first line treatment of metastatic microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer in adults. DOSAGE AND ADMINISTRATION See SmPC for full details. Therapy must be initiated and supervised by specialist physicians experienced in the treatment of cancer. The recommended dose of KEYTRUDA as monotherapy in adults is either 200 mg every 3 weeks or 400 mg every 6 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as monotherapy in paediatric patients aged 3 years and older with cHL is 2 mg/kg bodyweight (up to a maximum of 200 mg), every 3 weeks administered as an intravenous infusion over 30 minutes. The recommended dose of KEYTRUDA as part of combination therapy in adults is 200 mg every 3 weeks administered as an intravenous infusion over 30 minutes. KEYTRUDA must not be administered as an intravenous push or bolus injection. When administering KEYTRUDA as part of a combination with intravenous chemotherapy, KEYTRUDA should be administered first. Treat patients until disease progression or unacceptable toxicity. Atypical responses (i.e., an initial transient increase in tumour size or small new lesions within the first few months followed by tumour shrinkage) have been observed. Recommended to continue treatment for clinically stable patients with initial evidence of disease progression until disease progression is confirmed. For the adjuvant treatment of melanoma, KEYTRUDA should be administered until disease recurrence, unacceptable toxicity, or for a duration of up to one year. KEYTRUDA, as monotherapy or as combination therapy, should be permanently discontinued (a) For Grade 4 toxicity except for: endocrinopathies that are controlled with replacement hormones; or haematological toxicity, only in patients with cHL in which KEYTRUDA should be withheld until adverse reactions recover to Grade 0-1; (b) If corticosteroid dosing cannot be reduced to ≤10 mg prednisone or equivalent per day within 12 weeks; (c) If a treatment-related toxicity does not resolve to Grade 0-1 within 12 weeks after last dose of KEYTRUDA; (d) If any event occurs a second time at Grade ≥ 3 severity. Patients must be given the Patient Alert Card and be informed about the risks of KEYTRUDA. Special populations Elderly: No dose adjustment necessary. Data from patients ≥ 65 years are too limited to draw conclusions on cHL population. Data are limited in patients ≥ 75 years for pembrolizumab monotherapy in patients with resected Stage III melanoma and MSI-H or dMMR CRC; for pembrolizumab in combination with axitinib in patients with advanced RCC; for chemotherapy combination in patients with metastatic NSCLC; for pembrolizumab (with or without chemotherapy) in patients receiving first line treatment for metastatic or unresectable recurrent HNSCC. Renal impairment: No dose adjustment needed for mild or moderate renal impairment. No studies in severe renal impairment. Hepatic impairment: No dose adjustment needed for mild hepatic impairment. No studies in moderate or severe hepatic impairment. Paediatric population: Safety and efficacy in children below 18 years of age not established except in paediatric patients with cHL. CONTRAINDICATIONS Hypersensitivity to the active substance or to any excipients. PRECAUTIONS AND WARNINGS Assessment of PD-L1 status When assessing the PD-L1 status of the tumour, it is important that a well-validated and robust methodology is chosen to minimise false negative or false positive determinations. Immune-related adverse reactions Immune-related adverse reactions, including severe and fatal cases, have occurred in patients receiving pembrolizumab. Most immune-related adverse reactions occurring during treatment with pembrolizumab were reversible and managed with interruptions of pembrolizumab, administration of corticosteroids and/or supportive care. Immune-related adverse reactions have also occurred after the last dose of pembrolizumab. Immune-related adverse reactions affecting more than one body system can occur simultaneously. See SmPC for full details. Immune-related pneumonitis: Patients should be monitored for signs and symptoms of pneumonitis. Suspected pneumonitis should be confirmed with radiographic imaging and other causes excluded. Refer to SmPC for information on management of immune-related pneumonitis. Immune-related colitis: Patients should be monitored for signs and symptoms of colitis, and other causes excluded. Consider the potential risk of gastrointestinal perforation. Refer to SmPC for information on management of immune-related colitis. Immune-related hepatitis: Patients should be monitored for changes in liver function (at the start of treatment, periodically during treatment and as indicated based on clinical evaluation) and symptoms of hepatitis, and other causes excluded. Refer to SmPC for information on management of Immune-related hepatitis. Immune-related nephritis: Patients should be monitored for changes in renal function, and other causes of renal dysfunction excluded. Refer to SmPC for information on management of immune-related nephritis. Immune-related endocrinopathies: Severe endocrinopathies, including adrenal insufficiency, hypophysitis, type 1 diabetes
mellitus, diabetic ketoacidosis, hypothyroidism, and hyperthyroidism have been observed with pembrolizumab treatment and patients should be monitored for these endocrinopathies. Refer to SmPC for information on management of immune-related endocrinopathies. Immune-related skin adverse reactions: Patients should be monitored for suspected severe skin reactions and other causes should be excluded. Cases of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in patients receiving pembrolizumab. If SJS or TEN is confirmed, pembrolizumab should be permanently discontinued. Other clinically significant immune-related adverse reactions: The following additional clinically significant, immune-related adverse reactions, have been reported in clinical studies or in post-marketing experience: uveitis, arthritis, myositis, myocarditis, pancreatitis, Guillain-Barré syndrome, myasthenic syndrome, haemolytic anaemia, sarcoidosis, encephalitis, myelitis and vasculitis. Refer to SmPC for information on management of significant immune-related adverse reactions. Complications of allogeneic Haematopoietic Stem Cell Transplant (HSCT): Allogeneic HSCT after treatment with pembrolizumab: Cases of graft-versus-host-disease (GVHD) and hepatic veno-occlusive disease (VOD) have been observed in patients with classical Hodgkin lymphoma undergoing allogeneic HSCT after previous exposure to pembrolizumab. Allogeneic HSCT prior to treatment with pembrolizumab: In patients with a history of allogeneic HSCT, acute GVHD, including fatal GVHD, has been reported after treatment with pembrolizumab. Infusion-related reactions: For Grades 3 or 4 infusion reactions including hypersensitivity and anaphylaxis, stop infusion and permanently discontinue pembrolizumab. With Grades 1 or 2 infusion reactions, infusion may continue with close monitoring. Premedication with antipyretic and antihistamine may be considered. Overdose: There is no information on overdose with pembrolizumab. In case of overdose, monitor closely for signs or symptoms of adverse reactions and treat appropriately. INTERACTIONS No formal pharmacokinetic drug interaction studies have been conducted with pembrolizumab. No metabolic drug-drug interactions are expected. The use of systemic corticosteroids or immunosuppressants before starting pembrolizumab should be avoided because of their potential interference with the pharmacodynamic activity and efficacy of pembrolizumab. Corticosteroids can be used as premedication, when pembrolizumab is used in combination with chemotherapy, as antiemetic prophylaxis and/or to alleviate chemotherapy-related adverse reactions. FERTILITY, PREGNANCY AND LACTATION Women of childbearing potential Women of childbearing potential should use effective contraception during treatment with pembrolizumab and for at least 4 months after the last dose of pembrolizumab. Pregnancy No data on use in pregnant women. Do not use during pregnancy unless the clinical condition of the woman requires treatment with pembrolizumab. Breast-feeding It is unknown whether pembrolizumab is secreted in human milk. A risk to newborns/ infants cannot be excluded. Fertility No clinical data available. SIDE EFFECTS Refer to SmPC for complete information on side effects. Pembrolizumab is most commonly associated with immune-related adverse reactions. Most of these reactions resolved with appropriate medical treatment or withdrawal of pembrolizumab. The most serious adverse reactions were immune-and infusion-related adverse reactions. Monotherapy: Very Common: anaemia, hypothyroidism, decreased appetite, headache, dyspnea, cough, abdominal pain, nausea, vomiting, constipation, musculoskeletal pain, arthralgia, asthenia, oedema, pyrexia, diarrhoea, rash, pruritus, fatigue. Common: pneumonia, thrombocytopenia, lymphopenia, neutropenia, hyponatraemia, hypokalaemia, hypocalcaemia, insomnia, neuropathy peripheral, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, hyperthyroidism, thyroiditis, insomnia, dizziness, dysgeusia, pneumonitis, colitis, dry mouth, severe skin reactions, vitiligo, dry skin, alopecia, eczema, dermatitis acneiform, dermatitis, erythema, myositis, pain in extremity, arthritis, influenza like illness, chills, AST and ALT increases, hypercalcaemia, increase in blood alkaline phosphatase, blood bilirubin increased, blood creatinine increased, infusion related reaction. Combination with chemotherapy: Very Common: anaemia, neutropenia, thrombocytopenia, hypokalaemia, decreased appetite, dizziness, neuropathy peripheral, dysgeusia, headache, dyspnoea, cough abdominal pain, alopecia, diarrhoea, nausea, vomiting, constipation, rash, pruritus, musculoskeletal pain, arthralgia, pyrexia, fatigue, asthenia, oedema, blood creatinine increased. Common: pneumonia, febrile neutropenia, leukopenia, lymphopenia, infusion related reaction, hypothyroidism, hyperthyroidism, hyponatraemia, hypocalcaemia, insomnia, lethargy, dry eye, cardiac arrhythmia (including atrial fibrillation), hypertension, pneumonitis, colitis, dry mouth, severe skin reactions, erythema, dry skin, myositis, pain in extremity, arthritis, nephritis, acute kidney injury, chills, influenza-like illness, hypercalcaemia, ALT increase, AST increased, blood alkaline phosphatase increased. Combination with axitinib: Very Common: hyperthyroidism, hypothyroidism, decreased appetite, headache, dysgeusia, hypertension, dyspnoea, cough, dysphonia, diarrhoea, abdominal pain, nausea, vomiting, constipation, palmar-plantar erythrodysaesthesia syndrome, rash, pruritus, musculoskeletal pain, arthralgia, pain in extremity, fatigue, asthenia, pyrexia, alanine aminotransferase increased, aspartate aminotransferase increased, blood creatinine increased. Common: pneumonia, anaemia, neutropenia, leukopenia, thrombocytopenia, infusion related reaction, hypophysitis, thyroiditis, adrenal insufficiency, hypokalaemia, hyponatraemia, hypocalcaemia, insomnia, dizziness, lethargy, neuropathy peripheral, dry eye, cardiac arrhythmia (including atrial fibrillation), pneumonitis, colitis, dry mouth, hepatitis, severe skin reactions, dermatitis acneiform, dermatitis, dry skin, alopecia, eczema, erythema, myositis, arthritis, tenosynovitis, acute kidney injury, nephritis, oedema, influenza like illness, chills, blood
alkaline phosphatase increased, hypercalcaemia, blood bilirubin increased. PACKAGE QUANTITIES KEYTRUDA 25 mg/mL: 4 mL of concentrate in a 10 mL Type I clear glass vial. Legal Category POM. Marketing Authorisation numbers EU/1/15/1024/002. Marketing Authorisation holder Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands. Date of revision: March 2021. © Merck Sharp & Dohme B.V. 2021. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 or from www.medicines.ie. Date of Preparation: May 2021. II/090. Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie. Adverse events should also be reported to MSD (Tel: 01-2998700) References 1. Keytruda Summary of Product Characteristics, May 2021, available at www.medicines.ie. IE-KEY-00382 Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 Ireland
* Reimbursed as of May 1st 2021 on the Oncology Drugs Management System
5057_KEYTRUDA_Reimbursement5_Ad_250_346_v.indd 1 17/05/2021 16:28
HSE audit committee discussed ‘cyber risks’
DAVID LYNCH AND NIAMH CAHILL
Cyber security was on the agenda of the HSE’s audit and risk committee in March, weeks before the major ransomware attack that paralysed the health service.
The committee discussed “cyber risks” and sought a general overview of the HSE’s “technological landscape and changing risk profile”, according to minutes seen by the Medical Independent (MI)
The committee’s March meeting discussed “rapid ICT deployments and changing risk profiles” and heard that the “risks over the last year have changed with a rise in focus on cyber risks”.
The committee requested a follow-up session involving the HSE Internal Audit Division and Office of the Chief Information Officer to further discuss the issue.
Because of an error in uploading to the HSE website, the minutes of the March meeting are not complete.
Cyber security is one of 17 ‘red’ risks on the HSE cor-
Pregabalin and gabapentin ‘should be controlled’

CATHERINE REILLY
porate risk register (CRR) approved by the HSE board.
“There is a risk to the HSE effectively protecting the confidentiality, availability and integrity of HSE data including patient data against cyber threats impacting directly on patient care and safety and staff as a result of the inability to deliver ICT and specialised medical device dependent services,” reads the CRR.
Meanwhile, the National Coordinator of the general practice information technology group, Dr Conor O’Shea, told MI it was generally accepted that Ireland spent much less on healthcare IT than other jurisdictions.
Commenting on whether the attack could delay the introduction of electronic patient summaries, a unique health identifier and other IT projects, Dr O’Shea argued that the attack should not be allowed to result in further delays.
More IT investment is required and lessons must be learned from this incident, he said.
Expert group formed on breast implant-associated lymphoma
CATHERINE REILLY
The HSE has formed an expert advisory group on breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) to progress actions including an implant registry. Its first meeting was held in April.
BIA-ALCL is considered a rare and generally slow-developing type of non-Hodgkin’s lymphoma in individuals with breast implants, particularly those with a textured surface. Common symptoms include swelling in the area of the implant; substantial change in the size of the affected breast, which develops rapidly; and discomfort.
The condition is usually curable when identified and treated promptly. The Health Products Regulatory Authority said it had received “less than five” reports to date.
As of mid-February 2020, 348 patients had requested follow-up appointments through their cancer centre arising from concerns about BIA-ALCL. “All cancer centres accommodated patients through current
Pregabalin and gabapentin should be made controlled drugs in Ireland, according to the outgoing Medical Council President.
Dr Rita Doyle said addiction to pregabalin – also known by the brand name Lyrica – is “a huge issue” in this country.
“They have now made them controlled drugs in the UK, and I certainly foresee that will happen here,” she said.
In September 2019, the Medical Council warned it would take disciplinary action against doctors in relation to over-prescribing of benzodiazepines, z-drugs, and pregabalin. While the warning was believed to relate to a minority of doctors, a significant number of total complaints involve prescribing practice.
In recent years, regulatory action has been taken against a small number of doctors on this issue.
Dr Doyle said the Council’s over-prescribing working group is also discussing prescription of opiates, which became an epidemic in the US.
“And we are a little bit concerned that guidelines need to be more clarified, looking at what are the gold standards in medication and trying to encourage doctors to stick to the gold standards. There is a lot of advertising among the producers of these drugs.”
The Council has collaborated with the HSE, and other bodies, to produce resources for prescribers to raise public awareness of the potential harms associated with benzodiazepines and z-drugs.
These resources were circulated to GPs by the HSE in September 2020, along with a letter from Dr Doyle “to support you in patient consultations regarding benzodiazepines, z-drugs, and pregabalin”. See news interview, p4-5.
resources,” stated the HSE.
Internationally and nationally, implant removal in non-symptomatic patients for the purpose of BIA-ALCL prophylaxis is not recommended.
In 2019, a HSE serious incident management team (SIMT) was established following the global withdrawal of Allergen’s Biocell macro and micro-textured implants. Allergan Biocell implants had been withdrawn from the European market in December 2018 on foot of safety actions by French authorities.
According to the HSE SIMT’s final report, most cases internationally have been associated with Allergan Biocell implants and tissue expanders.
As of February 2020, the HSE had identified 2,711 people with Allergan Biocell implants from its hospital inpatient data system.
The SIMT initiated a number of actions including communications with patients and clinicians. Private hospitals were advised to contact their patients.
THE MEDICAL INDEPENDENT | 31 MAY 2021 3 News
Right-touch regulation
Credited as helping to improve the reputation of the Medical Council among doctors, GP Dr Rita Doyle is finishing her term as President. She spoke to Catherine Reilly about an eventful three years
Dr Rita Doyle’s term as Medical Council President began at the height of the CervicalCheck controversy. It concludes amid a major cybersecurity attack on the HSE. In between, the biggest health crisis in living memory paralysed much of the globe and placed an enormous strain on healthcare staff.

The ransomware attack was “a very cruel blow”, Dr Doyle told the Medical Independent (MI). It erupted just as healthcare staff were “beginning to think a bit of normality might be returning”.
With delayed or limited access to diagnostics and blood tests, for example, MI asked about potential regulatory implications for doctors. Dr Doyle said the Medical Council’s approach was “that all complaints are contextualised” – a position it has also voiced during the Covid-19 pandemic.
“And if you are finding yourself in a very awkward situation, just to be very clear about your documentation.” She said doctors’ notes should reference the cyberattack.
The situation has compounded fears about delayed diagnoses and late presentations due to the pandemic, according to Dr Doyle.
“It just couldn’t have happened at a worse time, it just couldn’t have,” reflected the Bray GP.
First woman
Appointed to the Medical Council in 2013 as the ICGP’s representative, Dr Doyle was elected Council President in July 2018, becoming the first woman to hold this role.

Dr Doyle has been a particularly vocal President, partly through her presence on Twitter. She has consistently highlighted issues in doctors’ working lives that impact on patient safety – excessive working hours, understaffing, bullying, communication breakdowns between primary and secondary care.
Doctors’ health has long been an interest. For the past eight years Dr Doyle has chaired the Council’s health committee, which supports doctors to maintain their registration during illness and/or disability (including addiction issues). Doctors can be referred, or self-refer, to the health committee.
Was there any change in scale or type of presentations during the pandemic?
“At the beginning, I think a couple of doctors who had mental health issues, they just found that Covid was the tipping point, but they got to us and we were able to support them and get them back to work,” Dr Doyle told MI
“The vast majority of doctors who come to us do get back to work, which is really good… I have a very fine committee, really stunning people who go out of their way to support these doctors, and it is something I am very proud of.
“Our numbers are now up to 50, but
that is the tip of the iceberg. Our register has nigh on 25,000 doctors, so if we have only 50 sick doctors within that, either we have a very healthy population or there are a lot of sick doctors who haven’t presented to us anyway.”
Dr Doyle added that the Council also has a memorandum of understanding with the independent Practitioner Health Matters Programme.
She said the health committee can help doctors in a unique way.
“It is not a therapeutic relationship, but we direct the care of these doctors, and I think it does a really good job, and I would like to see more doctors attending. The workload is heavy enough, each review team spends an hour with the doctor and they do at least two reviews a month.
“I think the remote access has actually made it easier for doctors, they don’t have to travel into Kingram House… they [previously] came in after hours and nobody would see them, anonymity and confidentiality was absolutely sound.”
Another matter raised prominently by Dr Doyle has been communication breakdowns between primary and secondary care, and vice versa. This issue is a major patient safety risk and a source of interprofessional tensions among doctors.
“We had a consultative forum, we have a document, and it is ready,” answered Dr Doyle on this matter. “I think the most appropriate place for this is probably Sláintecare, so I think it will probably be discussed with [Executive Director of the Sláintecare programme implementation office] Laura Magahy.
“I still feel very strongly about it. I think communication is still way below par and the transitional care – the journey of the patient from primary to secondary and back again – is the most dangerous journey the patient ever makes.”
The risks involve inadequate and delayed transfer of patient information between healthcare settings, particularly in
relation to prescribing.
“There is a screaming need for more clinical pharmacists both at community and at hospital level to help do the reconciliation of medications and also the question has to be asked – is it appropriate that the most junior doctor writes the discharge letter? And I think that question needs to be asked and answered by the profession.”
The document contains recommendations on “all parts” of the patient journey, according to Dr Doyle.
Over-prescribing
In September 2019, the Council issued a statement headlined ‘Medical Council warns doctors to reduce over-prescribing of benzodiazepines, z-drugs and pregabalin or face potential investigation’. It is understood the regulator did not consider poor prescribing practice as being widespread, but a significant amount of overall complaints related to prescribing.
Nevertheless, the tone of the Council’s press release attracted some ire within the profession. Dr Doyle said the statement was a reminder to doctors to review their prescribing practice. It was also intended to provide support to those who are being pressurised to prescribe these drugs.
“We made a statement about the guidelines for benzodiazepine prescribing to encourage doctors to stick with the guidelines, and that if they persistently voided the guidelines then we would take action.
“The logic behind our statement was to support doctors in being able to say to patients, ‘No I can’t, sure look, this is what the Medical Council has said to us, I can’t do it’.
“I am very aware that the act of not prescribing is much harder than the act of prescribing. It is much quicker to sign a prescription and it takes much longer not to prescribe, I am aware of that.”
dictive substances.”
The Council’s benzodiazepine working group has expanded to become an over-prescribing working group, outlined Dr Doyle.
“Of course we have now included opiates in our discussions. You know about the huge epidemic of opiate prescribing in the States and it literally spread from one side of the States to the other.
“And we are a little bit concerned that guidelines need to be more clarified, looking at what are the gold standards in medication and trying to encourage doctors to stick to the gold standards.
“There is a lot of advertising among the producers of these drugs… the oxycodone/ Oxycontin in the States was the big one, but there are other versions of that with things added to it… and not necessarily more efficacious medications appearing on the market all the time.
“So we are trying to get doctors to stick to the basic guidelines and follow gold standards. There will be a bit of education coming from that group.”
Dr Doyle believes pregabalin and gabapentin should be made controlled drugs in Ireland. She said addiction to pregabalin is a “huge” issue in this country.
“They have now made them controlled drugs in the UK, and I certainly foresee that will happen here. How long it takes I don’t know, but I certainly foresee that will happen.”
Undertakings
In recent years, the High Court President has made some sharp criticisms of the Council, including on its system where doctors make ‘undertakings’ in regard to their practice (for example, in some cases the Council accepts undertakings rather than initiating High Court proceedings to seek immediate suspension of a doctor’s registration).
In late 2019, the then High Court President Mr Justice Peter Kelly criticised the fact there was no system whereby employers/prospective employers could know a doctor had provided undertakings to the Council or to its health committee.
Mr Justice Kelly’s comments related to a case where a doctor had undertaken to the health committee in May 2016 not to practise medicine but was hired by a mental health service that was unaware of this information.
When MI spoke to Dr Doyle about the issue in October 2019, she said the High Court had recently temporarily prohibited a doctor from prescribing benzodiazepines following a process initiated by the Medical Council.
Since that time, Dr Doyle recalled at least three further cases.
“Obviously I cannot discuss specific cases, but certainly there were a couple of emergency meetings… where we applied to the High Court either to modify their prescribing or desist from prescribing ad-
Dr Doyle told MI the process has been revised such that, when undertakings are made to the Council or the health committee, the medical register notes that conditions are attached to the doctor’s practice.
“If you are an appropriate person to be asking the question, you can ring the Council and the information will be given to you,” stated Dr Doyle.
Complaints process
A major and long-awaited change is imminent as to how the Medical Council handles complaints on foot of legislative
THE MEDICAL INDEPENDENT | 31 MAY 2021
The HSE has shown no interest in GP health and that has to change
News Interview
CATHERINE REILLY catherine@mindo.ie
Dr Rita Doyle
amendments.
When the new procedures are operational, complaints will be first examined by the CEO/authorised officers rather than the preliminary proceedings committee (PPC). Complaints reaching a certain threshold (ie, those that concern a doctor’s fitness to practise (FTP)) will be investigated by the PPC.
“Now the work is ongoing on developing the policies and procedures associated with that,” outlined Dr Doyle.
Local processes for complaints in regard to GPs are being developed (this does not mean patients cannot still complain to the Council).
“We have more complaints about GPs than anybody else, but that is because there is more of us (GPs) and we see more patients,” noted Dr Doyle.
The Council has been working with the ICGP on developing a local complaints process supported by MPS and Medisec. Dr Doyle anticipates this will be in place by Christmas.
“The legislative change should be in process long before that. The professional standards department are working very hard on developing all of that, I would see that happening before September.”
The lay majority in the FTP committee
CervicalCheck, published in September 2018, recommended the Council update its ethical guide to ensure open disclosure was more clearly set out as a professional duty. However, this section has not yet been changed.
“No it is not, and there is still a lot of negotiation about the open disclosure,” Dr Doyle told this newspaper.
As recently reported by MI, draft legislation sets out mandatory disclosure of serious patient safety incidents. However, there have been concerns expressed by doctors about what they consider as the “bureaucratic” nature of the processes and unclear delineation between expected and unexpected complications.
As of mid-May, a revised draft HSE national open disclosure policy was due to be circulated for consultation.
Dr Doyle indicated the pandemic had stymied work on this issue.
However, she added: “If you go back to CervicalCheck, the patient’s information belongs to the patient and I think open and honest and frank disclosure is absolutely appropriate and if you make a mistake, to put your hand up and say, ‘look I am really sorry this is what happened and this is what I am going to do so it doesn’t happen again’. We all make mistakes; we are all human.”
the potential role of doctors.
Dr Doyle said the Council has not yet made any submissions on the Dying with Dignity Bill 2020. “We will watch what goes on and comment when we need to. We will have to stick with our very clear ethical guide.”
When asked, Dr Doyle said her personal view did not matter. “I believe, in general, the vast majority of patients can be looked after with great dignity and with good palliative care.”
Dr Doyle was the first female President of the Council and will be succeeded on 1 June by another woman – Paediatric Intensivist Dr Suzanne Crowe.
Recent discussions on social media highlighted the fact that female GPs must find, and in some cases finance, their own maternity cover. What further improvements are required to ensure female doctors can continue to progress their careers?
“I am laughing because I am that old I had no maternity leave. Neither paid or unpaid,” responded Dr Doyle.
“I would have been back to work six days after my youngest child was born. So that is appalling. It is a bit better today, but actually women should be treated like everybody else in the workforce, they are entitled to their statutory maternity leave and yes, the HSE should be providing cover for [female GPs on maternity leave].
“Because we are not HSE employees they have always held us at an arm’s distance, we are independent contractors, so as far as they are concerned you can die as long as you provide the cover, and that is wrong, it just has to change, full stop. The HSE has shown no interest in GP health and that has to change – that is not a Medical Council issue, that is a GP issue.
“But yes, women in the medical workforce should be treated exactly the same as women in all workforces, they should get their proper maternity leave and their cover should be arranged, full stop.”
MINDO NUMBERS
83
per cent of homeless people in their 20s, 30s, and 40s had mobility problems, found a study by researchers at Trinity College Dublin and St James’s Hospital, Dublin, which was published in Scientific Reports.
38 per cent of participants in the study could walk for six minutes and the majority (70.5 per cent) were frail or pre-frail.
€6m
investment for clinical trial networks was announced by the Health Research Board on the eve of International Clinical Trials Day 2021 (20 May).
86
was a major feature of the Medical Practitioners Act 2007. However, some patient advocates have highlighted that the PPC is dominated by doctors (the Act prescribes that all the Council’s committees, except for the FTP committee, should comprise a majority of doctors).
Dr Doyle said she would have no “emotional or moral objection” to a lay majority in the PPC, but believed this would not be practical. She said the PPC assessed a large number of complaints and medical expertise was important in this context.
“It is to do with the technical nature of the complaint and the knowledge base. And we have obstetricians, physicians and surgeons, every different type, and they are not all members of the Council, they are external members of the Council. But you need… a degree of medical expertise, you can’t go and get an expert on every case, you’d never get the work done. You definitely do need the medical expertise, I would think.”
Interestingly, it was also Dr Doyle’s general experience that “the lay people are kinder to the doctor… doctors can be very mean to doctors, for want of a better word”.
Ethical guide
A major review of the Council’s ethical guide is ongoing. The regulator has informed MI this process will conclude in 2023.
Dr Gabriel Scally’s scoping inquiry into
Social media
The ethical guide’s section on doctor’s use of social media will also be analysed. Asked if most doctors used social media appropriately, Dr Doyle said “my general impression is yes”.
“The vast majority of doctors behave in a very professional and correct manner. Of course, we hear more about the ones who stray than about the ones who don’t stray. But, it is my personal experience that most of the time that they do behave in a very professional manner and there are guidelines in the ethical guide on how to behave on social media.
“I would say if you identify yourself as a doctor on social media, then you have a responsibility – the same responsibility to be ethically correct as you would have if you were talking to somebody. The ethical principles must stay there; mud-slinging and nasty stuff is not appropriate.”
MI referred to a phenomenon whereby some doctors are promoting products on social media as part of commercial relationships.
This would be “a conflict of interests”, according to Dr Doyle. “That is a no-no… if you identify as a doctor you must behave in the same way as if the patient was sitting in front of you.”
The ethical guide’s last update occurred in 2019 following the enactment of abortion legislation. The next divisive issue for the profession could be assisted death and
According to Dr Doyle, the “powers that be never took any recognition about the feminisation of medicine”.
Covid-19
During the Covid-19 pandemic, Dr Doyle reflected that the Council had undertaken a “huge amount” of good work, including the swift re-registration of hundreds of doctors onto the medical register.
“Good work in supporting doctors, good work in keeping the channels of communication wide open, good work like working with the Department of Health, HSE and the Pharmaceutical Society to enable the electronic transfer of prescriptions…,” commented Dr Doyle.
Overall, Dr Doyle said she was “very proud” of the response of the Council, its staff and executive during this major public health crisis.
“We issued statements over the period of time. I think and hope the profession believed that we had their back, that we knew they were in very difficult situations, and that if complaints were made that they would be contextualised.”
The Council President reflects that it has been “a very challenging” three years at the helm.
“It has been a privilege, I have learned an enormous amount, I have worked with some stunning people.… I have had moments of panic, but I have enjoyed it. I just hope I did a half decent job.”
per cent of people want laws against coercive control expanded to cover all circumstances in which the abuse occurs, according to research commissioned by Safeguarding Ireland.
45 per cent said they would be able to identify coercive control, found the same research.
39
per cent would not know who to contact if they needed advice about helping someone experiencing coercive control.
84
consultant grade posts in public health have been promised in a deal approved by IMO members earlier this month.
THE MEDICAL INDEPENDENT | 31 MAY 2021 5 Interview News
I think and hope the profession believed that we had their back, that we knew they were in very difficult situations
Two major reviews by HSE-founded ‘independent panel’
CATHERINE REILLY

A national review panel established by the HSE to independently examine the most serious incidents in social care has completed two reports to date.
The national independent review panel (NIRP) has finalised two major reviews into serious events where an incident management team recommended an “independent review”, according to the HSE.
Comprised of social care professionals who do not work for the HSE, the NIRP was established in 2017 and is overseen by independent Chairperson, Ms Bernie McNally. A qualified social worker, Ms McNally has worked in a variety of practice and management positions in Northern Ireland’s social care services.
The panel was initially set up to examine serious cas-

es of concern in disability services, but has since become involved in other domains, including mental health and older persons services. The NIRP monitors the national incident management system to ensure all relevant incidents are considered for referral. Draft review plans are approved by the HSE National Director for Quality Assurance and Verification.
“Although the NIRP is part of the HSE it is independent of all HSE operations at both national and community level,” outlines the Executive.
“The NIRP will review cases where it is suspected that there are serious failings by the HSE and/or its funded organisations that have led to significant harm and/or have compromised the quality-of-life of the person/s concerned.
“The NIRP will seek to determine what the relevant services and individuals involved in the case might have

Plan to provide FMT in Ireland halted by pandemic
DAVID LYNCH
A plan to provide a national faecal microbiota transplant (FMT) service in Ireland was closed in late March after significant disruption caused by the Covid-19 pandemic, the Medical Independent (MI) can report.
The development of a new business case on what is required to “establish and maintain” a stool bank in Ireland is now underway.
In January 2020, MI reported that Stool Bank Ireland aimed to start providing FMT in Irish hospitals to treat refractory or recurrent Clostridioides difficile infection (CDI).
Stool Bank Ireland is a team of clinicians and researchers from a number of different organisations and the group received funding under the Sláintecare Integration Fund.
FMT involves the transfer of stool from a healthy donor into the patient’s gastrointestinal tract for the purpose of treating recurrent CDI.
Covid-19 “most certainly impacted the project”, Dr Fidelma Fitzpatrick, Consultant/Senior Lecturer in Microbiology at the RCSI and member
VOX BOX
of Stool Bank Ireland, recently told MI
Dr Fitzpatrick said these difficulties were not confined to this island.
“It also impacted FMT and stool banks worldwide,” she added.
“Following difficulties encountered sourcing a supply... it became apparent over the course of the grant that it was not possible to source FMT from overseas and that we would need to produce FMT in Ireland.”
There were plans to set up a facility in Cork to produce FMT for national use.
“This process would have extended beyond the lifetime of the grant, so we sought to lay the groundwork for this under the Sláintecare grant.
“However, it was not possible to do this under the grant, hence we had to close the project on 31 March 2021,” said Dr Fitzpatrick
However, Dr Fitzpatrick said the Department of Health has advised the group to prepare a business case outlining requirements to establish and maintain a stool bank in Ireland. This is currently in preparation. See news feature, p14.
done differently that could have prevented the significant harm or improved the quality-of-life for the person/s concerned.”
Its first review was submitted to the HSE in 2018. The report was not releasable under Freedom of Information (FoI) law due to a High Court order, according to the HSE. The subject of the report was a ward of court.
A second review completed by the NIRP has been submitted to the HSE. The report is “currently subject to the deliberative processes of the HSE” and access was refused under FoI law. This review relates to a HSE-provided disability service.
According to the NIRP’s annual report 2019-August 2020, it was completing a further three reviews.
The pandemic has impacted the capacity of services to engage with the NIRP, stated the HSE’s response under FoI.
Consultant private activity below 20 per cent threshold
PAUL MULHOLLAND
The private activity conducted by public hospital consultants was below the 20 per cent threshold specified in the consultant contract, according to the latest annual report prepared by the HSE on this issue, the Medical Independent can report.
The 2019 report, which was completed in August of last year, stated that 13.9 per cent of consultant workload related to private activity and was evidence of general adherence to the limits.
In 2018, the percentage was 15.4 per cent.
No cases of consultant non-compliances were escalated to the National Director of Acute Operations Mr Liam Woods in 2019.
A review of non-compliance with public/private mix has highlighted a number of factors, which are largely outside of the control of hospitals or consultants, and can contribute to overall levels of public/ private activity. Foremost among these was emergency admissions. In these cases, the consultant on-call was assigned the patient irrespective of the patient’s insurance status.
Other factors included: The specialist services that the hospital provides; wheth-
er it is a larger hospital providing emergency and complex care or a smaller hospital with less complex emergency, day case and elective care; whether comparable services are available in a nearby private hospital; where there is no private hospital located in the same region as the public hospital; and the various consultant contract types within the hospital.
In January 2020 the HSE’s Internal Audit Division reported to Mr Woods on the effectiveness of the consultant contract compliance framework. The report included 10 findings and 13 related recommendations. At time of completion of the 2019 report, six recommendations were fully implemented, while seven were in in progress.
The issue of monitoring contract compliance was discussed at a meeting of the HSE’s audit and risk committee on 9 October 2020. Mr Woods spoke about compliance framework and audit findings. The committee noted Mr Woods’s comments “on the delays that Covid has caused and that paradoxically compliance may have risen during this time; however, breaches could still be observed”.
See news feature, p10.
6 THE MEDICAL INDEPENDENT | 31 MAY 2021 News
The cyberattack has had a profound effect on healthcare. Without the support of IT, some care will be slower and delays inevitable. Thank you to patients for understanding and to teams striving to provide safe care in difficult circumstances.”
HSE Chief Clinical Officer Dr Colm Henry on the effect of the recent cyberattack on health services.
The disgraceful cyberattack this week is an attack on the Irish State and all of us who value our health system so highly, particularly in light of the heroic response from all members of our health services in the last year.”
After all that our health service has been through with Covid-19, everyone is gutted and angry with the impact of this cyber attack. It’s unfair, unjust, and incomprehensible. But the resolve in the HSE is incredibly strong to rebuild. It will take time, but we will do so.”
HSE CEO Mr Paul Reid on the cyberattack.
Dr Fidelma Fitzpatrick
Mr Liam Woods
Minister for Health Stephen Donnelly commenting on 20 May after the granting of a court order against the illegal use of data that may have been stolen during the cyberattack.
Genuair®-has it ‘clicked’ yet?

The ONLY prefilled inhaler with visual and audible feedback for confirmed dose delivery1-4

Genuair - a simple to use inhaler for patients with COPD4

Abbreviated Prescribing Information
Eklira® Genuair® 322 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and a green dosage button. Each delivered dose contains 375 µg aclidinium bromide equivalent to 322 µg of aclidinium. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).

Dosage: For inhalation use. Recommended dose is one inhalation of 322 micrograms aclidinium twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work differently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to aclidinium bromide or to any of the excipients. Warnings and Precautions: Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Use with caution in patients with symptomatic prostatic hyperplasia or bladder-neck obstruction or with narrow-angle glaucoma. Do not use in patients with rare hereditary problems of galactose intolerance, total lactose deficiency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic-containing medicinal products. No other interactions expected. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Risk to newborns/infants cannot be excluded. Consider risk-benefit before using during lactation. Unlikely to affect fertility at the recommended dose. Side-effects: Common (1-10%): Sinusitis, nasopharyngitis, headache, cough, diarrhoea, nausea. Uncommon (0.1-1%): Dizziness, blurred vision, tachycardia, palpitations, dysphonia, dry mouth, stomatitis, rash, pruritus, urinary retention. Rare (0.01-0.1%): hypersensitivity. Not known: angioedema, anaphylactic reaction. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing Authorisation Number: EU/1/12/778/002
Marketing Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: February 2020
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions to: HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744.
Date of item: November 2020. IR-BRI-09-2020
Abbreviated Prescribing Information Brimica® Genuair® 340 micrograms/12 micrograms inhalation powder. Please consult the Summary of Product Characteristics (SPC) for the full prescribing information. Presentation: Inhalation powder in a white inhaler with an integral dose indicator and an orange dosage button. Each delivered dose contains 396 µg aclidinium bromide (equivalent to 340 µg of aclidinium) and 11.8 micrograms of formoterol fumarate dihydrate. Also, contains lactose. Use: Maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Dosage: For inhalation use. Recommended dose is one inhalation of 340 µg/12 µg twice daily. Patients should be instructed on how to administer the product correctly as the Genuair inhaler may work differently from inhalers used previously. It is important to instruct the patients to read the Instructions for Use in the pack. No dose adjustments are required for elderly patients, or those with renal or hepatic impairment. No relevant use in children and adolescents. Contraindications: Hypersensitivity to the active substances or to any of the excipients. Warnings and Precautions: Do not use in asthma. Stop use if paradoxical bronchospasm occurs and consider other treatments. Do not use for the relief of acute episodes of bronchospasm. Use with caution in patients with myocardial infarction in the previous 6 months, unstable angina, newly diagnosed arrhythmia within the previous 3 months, or hospitalisation within the previous 12 months for heart failure functional classes III and IV. Discontinue if increases in pulse rate, blood pressure or changes in ECG occur. Use with caution in patients with a history of or known prolongation of the QTc interval or treated with products affecting the QTc interval. Use with caution in patients with severe cardiovascular disorders, convulsive disorders, thyrotoxicosis and phaeochromocytoma. Hypokalaemia may occur, is usually transient and supplementation not needed. In patients with severe COPD, hypokalaemia may be potentiated by hypoxia and concomitant treatment. Use with caution in patients with symptomatic prostatic hyperplasia, urinary retention or with narrow-angle glaucoma. Dry mouth, observed with anticholinergic treatment, may be associated with dental caries in the long term. Do not use in patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption. Interactions: Do not administer with other anticholinergic and/or long-acting β2-adrenergic agonist containing medicinal products. Caution in use with methylxanthine derivatives, steroids, non-potassium-sparing diuretics, β-adrenergic blockers or medicinal products known to prolong the QTc interval. Please consult the SPC for more details. Fertility, pregnancy and lactation: No data on use in pregnancy. Consider risk-benefit before using during lactation. Unlikely to affect fertility at the recommended dose. Side-effects: Common (1-10%): Nasopharyngitis, urinary tract infection, sinusitis tooth abscess, insomnia, anxiety, headache, dizziness, tremor, cough, diarrhoea, nausea, dry mouth, myalgia, muscle spasms, peripheral oedema, increased blood creatine phosphokinase. Uncommon (0.1- 1%): Hypokalaemia, hyperglycaemia, agitation, dysgeusia, blurred vision, tachycardia, electrocardiogram QTc prolonged, palpitations, angina pectoris, dysphonia, throat irritation, stomatitis, rash, pruritus, urinary retention, increased blood pressure. Rare (0.01-0.1%): Hypersensitivity, bronchospasm, including paradoxical. Not known: anaphylactic reaction, angioedema. Pack sizes: Carton containing 1 inhaler with 60 unit doses. Legal category: POM Marketing

Authorisation Number: EU/1/14/963/001 Marketing Authorisation holder: AstraZeneca AB, SE-151 85 Södertälje, Sweden. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd., Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924. Further information is available on request to A. Menarini Pharmaceuticals Ireland Ltd. or may be found in the SPC. Last updated: October 2019
This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions via
Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@ hpra.ie. Adverse events should also be reported to A. Menarini Pharmaceuticals Ireland Ltd. Phone no: 01 284 6744. References: 1. MIMS Ireland November 2020 2. Eklira® Genuair® Summary of Product Characteristics, last updated November 2019 3. Brimica® Genuair® Summary of Product Characteristics, last updated August 2019 4 Magnussen, H et al. COPD. 2019 Apr;16(2):196-205
HPRA Pharmacovigilance,
LAMA + LABA
LAMA
Comprehensive ‘hub and spoke’ hepatology network in development
NIAMH CAHILL
Work has begun on the development of a new ‘hub and spoke’ hepatology network aimed at improving patient access to care nationally, it has emerged.
Described by President of the Irish Society of Gastroenterology (ISG) Dr Tony Tham as a “work in progress”, the project would see the introduction of new care pathways to help provide a more comprehensive patient service.
Dr Tham, a Consultant Physician and Gastroenterologist at Ulster Hospital, Belfast, said the network would make hepatology care more accessible and create integrated care pathways for patients with gastro-intestinal diseases to access care for complaints such as liver failure and inflammatory bowel

Maintenance work required at State Lab following leaks
DAVID LYNCH
The Office of Public Works (OPW) has agreed the “budget and methodology” to carry out maintenance work at the State Laboratory building following leaks after heavy rain earlier this year, the Medical Independent (MI) can report.
At the February meeting of the State Laboratory management board, minutes of which have been seen by MI following a Freedom of Information request, it was mentioned there was “ingress in a number of labs” following heavy rain.
“Four labs were affected by this water ingress, work was not required to be halted and no damage to equipment occurred,” a spokesperson for the State Laboratory told MI
“The OPW has agreed budget and methodology to carry out maintenance work on the façade of the State Laboratory building.” The work was due to commence imminently.
“In the meantime, there will be additional surveillance of laboratories experiencing rainwater leaks during heavy rainfall out of hours and at weekends.”
At the November management board meeting, the installation of a tobacco analysis room and the commissioning of a smoking machine was discussed.
However, the spokesperson said “there have been delays in commissioning [tobacco analysis] room and instrument as a result of restricted work practices and travel for engineers during the pandemic”.
The State Laboratory was designated by the Department of Health as the testing laboratory for Ireland for the purposes of carrying out testing on tobacco products, as set out in the European Union (Manufacture, Presentation and Sale of Tobacco and Related Products) Regulations 2016.
A tobacco analysis room provides the necessary controlled temperature and humidity environment required for the correct operation of a smoking machine, which is required to test the emissions produced by tobacco products.
“A smoking machine is used to smoke tobacco products in order to test the tar, nicotine, and carbon monoxide content. Whilst no machine-smoking regimen can represent all human smoking behaviour, this machine smoking testing is useful for characterising cigarette emissions for design and regulatory purposes,” said the spokesperson.
disease, as well as nutrition, paediatrics, and some endoscopy services.
“For example, a central hospital would have a network arrangement with a smaller hospital so that patient treatment could be transferred from the smaller hospital to the larger hospital if required,” Dr Tham told the Medical Independent in advance of the Society’s Summer Meeting, which will be held on 18 June.
“The expertise from the central hospitals could also go out to the smaller hospitals and deliver local care.”
The network is being progressed by the HSE National Clinical Programme for Gastroenterology and Hepatology clinical advisory group, of which Dr Tham is Chairperson.
The clinical advisory group, set up by the RCPI, oversees the work of the HSE Clinical Programme for Gastroenterology and Hepatology, which is led by Prof Colm O’Moráin.
An agreed plan on the network will be devised before a business case is presented to the HSE for approval.
“We are making progress. A few years ago we started off with nothing and now we have a programme lead, in Colm
O’Moráin, who is bringing everyone together.”
Despite progress within the specialty, the pandemic has had a devastating impact and continues to present challenges for gastroenterologists.
According to Dr Tham, the disease has resulted in a huge backlog of gastroenterology cases, both North and South.
“On the whole island of Ireland our waiting lists are pretty terrible. There will be delayed diagnosis of cancer and patients with serious benign conditions being detected later. That’s the side-effect of Covid-19 on our patients.
“We’ve seen an increase in the referral rate since Covid and so with the increased referrals we are detecting more cancers as well. It’s hard to know what the impact would be if not for Covid, but you need a huge data set for that.
“I would anticipate that many years down the line, when they analyse the data, that cancer survival rates may dip. That’s speculation at the moment, but it makes sense if you detect cancers later that the prognosis would not be as good, but we don’t have the data yet to show that.”
See conference preview, p28-29.
HSE seeks Covid-19 swab collection service to potentially send samples overseas
PAUL MULHOLLAND
The HSE has gone to tender for the provision of a national collection and transportation service for Covid-19 specimen swabs, which includes the provision for samples to be sent overseas in the event on Irish laboratory services being overwhelmed.
The supplier will be required to collect Covid-19 specimen swabs and deliver them to HSE and third-party performing laboratories for processing, as directed by the HSE’s Covid laboratory operations and logistics team.
The competition consists of two lots. The first lot is for the collection and transport of Covid-19 specimen samples required from the various locations to specified community and third-party performing laboratories for processing.
These locations include: Residential care facilities; community test centres; workplaces; prisons; social care settings; quarantine facilities; isolation facilities; universities; pop-up centres; and acute hospitals/labs.
The HSE said that collection locations may be “activated” or “deactivated” based on demand.
The second lot is for collection and transportation of Covid-19 samples to a specified overseas laboratory.
“The [HSE] may require a national Covid-19 specimen
swab collection and transportation service to a specified overseas laboratory,” according to the tender documents.
“On occasion demand may exceed the laboratory test capacity onshore in Ireland. In this event it may be necessary to transport samples offshore for testing. Samples may be shipped to a number of laboratories across Europe (predominantly Germany and France) for processing at the specified overseas laboratories.
“Where possible the HSE will endeavour to provide the service provider with seven days’ notice in advance of the commencement of this requirement.”
In the event that support is required from overseas laboratories, the service provider will be required to transport designated samples from various locations as described in lot one to a nominated sample preparation site/laboratory, so that samples can be packed in preparation for offshore transportation.
Samples must be delivered to a preparation site within a pre-determined timeframe to allow adequate time for preparation and packing for onward transportation.
The samples would also need to be delivered on the same day to Dublin Airport.
The HSE’s preference would be for a single supplier “as this meets the optimum operational requirements and represents best value for money” for the Executive.
The deadline for tender submissions was 19 May.
6,700 HSE staff on Covid-related absence during one week in Jan
DAVID LYNCH
The level of HSE staff absence due to Covid-19 reached 6,700 in a single week in January, the Medical Independent (MI) can report. The median time of these Covid-related absences ranged from 12 to 14 days.
The issue of staff absence during the height of the pandemic was discussed at the February meeting of the HSE people and culture committee.
According to minutes of the February meeting, the committee was briefed on the “high levels” of staff absences due to Covid-19.
The committee was told that human resources leadership “locally and nationally have been challenged with providing services”, whilst managing the available staff resources.
“During one week in January, 6,700 staff were on Cov-
id-related absences. It was noted that with a median time of these Covid-related absences range from 12-to14 days,” stated the minutes.
According to the HSE, it was the largest employer in the State with more than 67,000 direct employees.
“The committee queried the effect of increased numbers of vaccinated staff on the rates of absences within the organisation and were informed that the biggest effect is to be expected when staff members begin to receive their second dose of the vaccine.”
In March, MI reported that the meeting of the HSE people and culture committee in January noted the “high rate of absences” among employees due to having Covid-19 or being a close contact.
The committee had discussed what the HSE could do to “support staff more adequately” and “advocate” on their behalf.
THE MEDICAL INDEPENDENT | 31 MAY 2021 News 8
Dr Tony Tham
Latest modules
Diagnosing and treating psoriasis
2 CPD Hours
Authors: Prof Anne-Marie Tobin, Consultant Dermatologist, Tallaght University Hospital, Clinical Associate Professor at Trinity College Dublin, and HSE Clinical Lead in Dermatology
Cardiac amyloidosisOverview, Diagnosis and Management
2 CPD Hours
Authors: Dr Neasa Starr, Advanced Heart Failure Registrar, and Prof Niall Mahon, Consultant Cardiologist, Mater University Hospital, Dublin
Diabetes Continuous glucose monitoring
2 CPD Hours
Authors: Dr Michael Lockhart, Endocrinology SpR, and Prof Diarmuid Smith, Consultant Diabetologist and Endocrinologist, Academic Department of Diabetes and Endocrinology, Beaumont Hospital and RCSI Medical School
A B C doctorCPD.ie Visit www.medilearning.ie/doctorcpd.ie Free CPD – now accessible on android, iPhone and tablet
Many factors driving public/private mix ‘outside control’ of consultants
Paul Mulholland examines recent HSE documents regarding its monitoring of the private activity carried out by consultants

In April 2018, the Department of Health requested that the HSE establish a framework to ensure compliance with the terms of the consultant contract regarding private practice.
The Department requested that the HSE’s Internal Audit Division be consulted on the establishment of the framework and that consultant compliance form part of the Executive’s annual internal controls process. It was also requested that the HSE’s audit committee be asked to include consultant contract compliance in its work programme.
The request was made shortly before the High Court settlement in June 2018 on monies owed under the consultant contract. The Department and HSE lost the case. The estimated cost of the settlement was €182 million for arrears and ongoing costs of €62 million per annum from 2019, backdated to the date of the settlement.
Before 2014, compliance with the contract had national oversight. However, in 2014 this was delegated to the Hospital Group CEOs. Monthly reporting by individual hospitals to the National Director of Acute Hospitals was no longer a requirement. The new framework was created to re-introduce oversight at a national level.
Through Freedom of Information legislation, the Medical Independent has obtained the 2019 annual report on the private practice of public hospital consultants and their adherence to private practice limits set in the consultant contract. The report, which was completed in August of last year, stated that a number of issues highlighted in the 2018 report had been addressed “in order to enable effective oversight”.
The 2018 report noted there were challenges in assessing compliance for consultants who were contracted in multiple sites because the ‘paymaster hospital’ was required to oversee compliance, but did not have access to the data for the other sites. Due to complexities relating to data-sharing, hospitals have now been instructed to report their own activity only, regardless of consultant discharges in other public hospitals.
“Subsequently, if a consultant in a hospital, who holds a split appointment over two or more hospital locations, reports a high ratio of private activity in one location his/ her activity should then be reviewed in the context of services provided by the consultant over all sites by combining the weighted units,” according to the 2019 report.
Treatment of locum consultants, in assessing overall compliance, was an open item at the time of the previous report. Since then, it has been agreed that locum consultants should be included in the process in the same way as consultants on contracts.
Completeness of hospital inpatient enquiry (HIPE) coding within 30 days on a number of sites was a challenge for many hospitals. This resulted in delayed reporting and amended data.
“This has been addressed with the Hospital Groups by improvements to the report templates generated by the Healthcare Pricing Office (HPO) and through training and communication with the hospitals,” according to the 2019 report.
The report stated HR/medical administration capability remained a challenge.
“However, familiarity with the reporting requirements and the data has meant greater levels of reporting compliance,” it stated.
The 2019 report said mechanisms are still required to include outpatient and diagnostic activity so that overall compliance can be measured more effectively. No effective mechanism has been identified to date.
“The expansion of the ABF [activity-based funding] model and the use of NIMIS [National Integrated Medical Imaging System] may offer opportunities to address this issue and this is being examined by Acute Operations in consul-
tation with CIO [Chief Information Officer] and HPO.”
Public/private mix
Internal audit
No consultant non-compliances were escalated to the National Director Acute Operations in 2019.
4.0 Internal Audit
According to the 2019 report, 13.9 per cent of patients seen by public hospital consultants were on a private basis. This was below the 20 per cent threshold set in the contract and is evidence of a "general adherence to limits" (see Table A for a breakdown per Hospital Group). In 2018, the percentage was 15.4 per cent.
In January 2020, the HSE’s Internal Audit Division reported to the National Director of Acute Operations on the effectiveness of the consultant contract compliance framework. The report included 10 findings and 13 related recommendations. At the time of the 2019 report’s completion, six recommendations were fully implemented, while seven were in in progress.
In Jan 2020 HSE Internal Audit reported to the National Director of Acute Operations on the effectiveness of the Framework for Reporting Consultant Contract Compliance (CCC). The report included 10 findings and 13 related recommendations. To date 6 recommendations are fully implemented and 7 are in progress. Those to be implemented include:
A review of non-compliance with public/private mix has highlighted a number of factors which are largely outside of the control of the hospitals or consultants, and can contribute to overall levels of public/private activity. Foremost among these is emergency admissions.
• Finalising the CCC Guidance Document.
“Emergency admissions account for most of the inpatient workload. In these cases the consultant on call is assigned the patient irrespective of the patient insurance status and therefore it is outside of the consultant’s control,” according to the report.
Those to be implemented include: Finalising the compliance guidance document; agreeing and implementing an approach to the measurement of outpatient and diagnostic activity (two recommendations); ensuring that the standard template for reporting is implemented across all Hospital Groups; ensuring work plans reflect Haddington Road Agreement commitment; annual declaration template to be drafted and reviewed by the Department of Health; and developing a detailed training module.
• Agree and implement an approach to the measurement of Outpatient and Diagnostic activity (two recommendations)
“To put this into context, 69 per cent of all inpatient discharges in 2019 were admitted on an emergency basis (2018 – 68 per cent).”
• Ensure that the standard template for reporting is implemented across all Hospital Groups.
• Ensure work plans reflect Haddington Road Agreement commitment.
Other factors include: The specialist services that the hospital provides; whether it is a larger hospital providing emergency and complex care or it is a smaller hospital with less complex emergency, day case and elective care; whether comparable services are available in a near-by private hospital; where there is no private hospital located in the same region as the public hospital all required private work will be carried out in the public hospital; and the various consultant contract types within the hospital.
• Annual Declaration Template to be drafted and reviewed by the Dept of Health.
• Development of a detailed training module
During 2019, HSE Internal Audit also carried out audits in seven acute hospitals. The audits were across four Hospital Groups and comprised three voluntary and four statutory hospitals. The main findings related to partial compliances in respect of work plan completion and processes for off-site compliance. The recommendations were all either implemented or in progress.
The issue of monitoring contract compliance was discussed at a meeting of the HSE’s audit and risk committee on 9 October 2020. The HSE National Director of Acute Operations Mr Liam Woods spoke about compliance framework and audit findings. The committee noted Mr Woods’s comments “on the delays that Covid has caused and that paradoxically compliance may have risen during this time; however, breaches could still be observed”.
The report also noted if consultants in the hospital hold split appointments in that they work over two or more hospital locations – a high ratio of private activity in one location may be offset by a low ratio in another location.
Data can appear skewed if the consultant in question only has a small number of discharges during a reporting period, while there are challenges relating to the application of the process for consultants on short-term contracts as the reporting and monitoring is performed three months in arrears in line with HIPE coded data availability. Completeness of HIPE coding can have an impact on the overall compliance reported.
“These factors are taken into consideration in determining whether intervention and escalation is required,” according to the report.
“There is active management of non-compliance and hospitals are actively engaging with consultants with a view to continuing improvements in this area.”
No cases of consultant non-compliances were escalated to the National Director of Acute Operations in 2019.
Mr Woods answered questions regarding the consultant recruitment process and “the extent at which managing the existing contracts will inhibit consultant recruitment going forward”.
“He stated that the option to do private work may be an attractor and this is not currently an inhibitor,” according to the minutes.
During 2019 HSE Internal Audit also carried out audits in seven acute hospitals. The audits were across four Hospital Groups and comprised three voluntary and four statutory hospitals. The main findings related to partial compliances in respect of work plan completion and processes for off -site compliance. The recommendations are all either implemented or in progress at the report date. Appendix
The committee noted that 29 type C contracts, which allow for private work off site, had recently been approved.
Mr Woods clarified that the measurement of diagnostic and outpatient activity is complex and noted that work is being undertaken with HIPE to better understand “the division of work in this area between the public and private space”.
“He highlighted that outpatients is not a particularly vulnerable area to consultant contract compliance, while the biggest residual area of exposure is in diagnostics due to complications in measurement.”
THE MEDICAL INDEPENDENT | 31 MAY 2021 News Feature
4
PAUL MULHOLLAND paul@mindo.ie
Table A. Submissions made by Hospital Groups relating to 2019 Compliance with Public Private Mix as at 31st of July 2020
* DMHG excludes the Coombe Women and Infants University Hospital . This has been escalated with the hospital by the Hospital Group.
1 InpatientDay Case Childrens Health Ireland Jan - Nov3 395 10.5%10.3% Dublin Midlands Hospital Group * Jan - Dec6/7 375 24.0%17.9% Ireland East Hospital Group Jan - Dec 10 950 6.6%4.1% RCSI Hospital Group Jan - Dec7 456 Saolta Hospital Group Jan - Dec6 441 15.1%15.9% South/South West Hospital Group Jan - Dec 10 486 26.3%31.8% UL Hospitals Group Jan - Dec6 225 25.3%26.5% Total 48 3328 20.8% Hospital Group % reported as not compliant 2019 Period Covered No of sites reported No of consultants included
Table A: Submissions made by Hospital Groups relating to 2019 compliance with public/private mix as of 31 July 2020
Significantly improves exercise duration and reduces angina frequency1

Abbreviated Prescribing Information: Ranexa (ranolazine). Please consult the Summary of Product Characteristics (SPC) for full prescribing information. Presentation: Prolonged-release tablets containing 375 mg, 500 mg or 750 mg of ranolazine. 750 mg tablet contains E102 and lactose. Use: Ranexa is indicated as add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled or intolerant to first-line antianginal therapies (such as beta-blockers and/or calcium antagonists). Dosage and administration: Oral administration. Patients should be instructed to list their medication to their health care professional at each visit. Adults: Initial dose is 375 mg twice daily. After 2-4 weeks, dose should be titrated to 500 mg twice daily and, according to patient’s response, further titrated to 750 mg twice daily. Concomitant treatment with moderate CYP3A4 and P-glycoprotein (P-gp) inhibitors: Careful dose titration is recommended. Renal impairment: Careful dose titration is recommended in mild to moderate renal impairment, and contraindicated in severe renal impairment. Hepatic impairment: Careful dose titration is recommended in mild hepatic impairment, and contraindicated in moderate to severe hepatic impairment. Elderly: Dose titration in the elderly should be exercised with caution. Low weight: Dose titration in patients with low weight should be exercised with caution. Congestive Heart Failure (CHF): Dose titration in moderate to severe CHF should be exercised with caution. Paediatric patients: No data in children below the age of 18 years. Ranexa tablets should be swallowed whole and not crushed, broken or chewed. They may be taken with or without food. Contra-indications: Hypersensitivity to the active substance or to any of the excipients. Severe renal impairment. Moderate or severe hepatic impairment. Concomitant administration of potent CYP3A4 inhibitors. Concomitant administration of Class Ia or Class III antiarrhythmics other than amiodarone. Warnings and Precautions: Caution should be exercised when prescribing or up titrating ranolazine to patients in whom an increased exposure is expected. QT prolongation: Caution should be observed when treating patients with a history of congenital or a family history of long QT syndrome, in patients with known acquired QT interval prolongation, and in patients treated with drugs affecting the QTc interval. Interactions: Co-administration with CYP3A4 inducers is expected to lead to lack of efficacy.
Renal impairment: Check renal function at regular intervals during treatment. Interactions: CYP3A4 inhibitors: Increase plasma concentrations of ranolazine. Combining ranolazine with potent CYP3A4 inhibitors is contraindicated. CYP3A4 inducers: Avoid initiation with Ranexa during administration of CYP3A4 inducers. CYP2D6 inhibitors: May increase plasma concentrations of ranolazine. Effect of ranolazine on other medicinal products: Dosage adjustment of sensitive CYP3A4 substrates and CYP3A4 substrates with a narrow therapeutic range may be required. Lower doses of CYP2D6 substrates may be required. Caution with CYP2B6 substrates. Monitor digoxin levels following initiation and termination of Ranexa. Limit dose of simvastatin to 20mg once daily in patients taking Ranexa. Limit dose of atorvastatin and consider clinical monitoring in patients taking Ranexa. Monitor blood levels of tacrolimus when coadministering with Ranexa and adjust tacrolimus dose accordingly. Also recommended for other CYP3A4 substrates with a narrow therapeutic range. Drugs transported by the Organic Cation Transporter-2 (OCT2): Plasma exposure of metformin increased in subjects with type 2 diabetes mellitus when co-administered with Ranexa. The exposure of other OCT2 substrates may also be affected. Theoretical risk that concomitant treatment with drugs known to prolong the QTc interval may increase the possible risk of ventricular arrhythmias. Pregnancy and lactation: Ranexa should not be used during pregnancy unless clearly necessary. Ranexa should not be used during breast-feeding. Effect on fertility unknown. Side-effects: Generally mild to moderate in severity and often develop within the first 2 weeks of treatment. Common (1-10%): dizziness, headache, constipation, vomiting, nausea, asthenia. Uncommon (0.1–1%): anorexia, decreased appetite, dehydration, anxiety, insomnia, confusional state, hallucination, lethargy, syncope, hypoaesthesia, somnolence, tremor, postural dizziness, paraesthesia, blurred vision, visual disturbance, diplopia, vertigo, tinnitus, hot flush, hypotension, dyspnoea, cough, epistaxis, abdominal pain, dry mouth, dyspepsia, flatulence, stomach discomfort, pruritus, hyperhydrosis, pain in extremity, muscle cramp, joint swelling, muscular weakness, dysuria, haematuria, chromaturia, fatigue, peripheral oedema, increased blood creatinine, increased blood urea, prolonged QT corrected interval, increased platelet or white blood cell count, decreased weight. In a long term study, acute renal failure was also reported with an incidence less than 1% in placebo and ranolazine patients. Rare (0.1-0.01%): hyponatremia, disorientation, amnesia, depressed level of consciousness, loss of consciousness, coordination abnormal, gait disturbance, parosmia, impaired hearing, peripheral coldness, orthostatic hypotension, throat tightness, pancreatitis, erosive duodenitis, oral hypoaesthesia, angioedema, allergic dermatitis, urticaria, cold sweat, rash, acute renal failure, urinary retention, erectile dysfunction, elevated levels of hepatic enzyme. Not known: myoclonus. Increased incidence of congestive heart failure and transient ischaemic attacks seen in patients with history of chronic angina who had incomplete revascularisation after percutaneous coronary intervention and treated within 2 weeks with ranolazine (1000 mg twice daily [dose not approved in Europe]) in a placebo-controlled post-PCI trial. Elderly, renal impairment and low weight: In general, adverse events occurred more frequently among elderly patients and patients with renal impairment. Adverse events in patients with low body weight were similar to those of patients with higher weight. Please consult the SPC for further information.Pack size: 60 tablets. Legal category: POM. Marketing authorisation numbers: EU/1/08/462/001, 003, 005 Marketing Authorisation holder: Menarini International Operations Luxembourg S.A. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd. Further information is available on request from A. Menarini Pharmaceuticals Ireland Ltd, 2nd Floor, Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924 or may be found in the SPC. Last updated: October 2020
Date of item: November 2020. IR-RAN-12-2020
References: 1. Chaitman, B.R., et al. JAMA, 2004; 291(3): p. 309-16.
Assessing President Biden’s first 100 days
During his crucial first 100 days in office, US President Joe Biden has touted his efforts to defeat Covid-19. But major challenges lie ahead that could threaten this progress. Bette Browne reports
American Presidents have been somewhat defined by how much of their agenda they managed to pass in their first 100 days in office. But never has a modern US leader’s success been measured by their ability to vanquish a disease. That is the daunting challenge facing new President Joe Biden.
President Biden is credited with a number of impressive wins in the battle against the Covid-19 pandemic since he took over the White House in January. But winning battles does not necessarily mean victory in his self-declared “war” on the disease. He is now facing roadblocks that will test his political mettle if he is to ultimately declare triumph.
Even before he took office, President Biden knew that Covid-19 would be his toughest enemy before attempting to implement other elements of his political agenda. “We’re in a war with this virus,” he declared during his election campaign to defeat former President Donald Trump, who arguably lost the White House because he played down the disease even as it raged across the country.

By contrast, President Biden’s war analogy was meant to focus the minds of Americans, many of whom rejected the need to wear masks or follow social distance rules.
On 21 January 2021, just one day after he took office, Covid-19 deaths in the US reached and then exceeded the 405,399 American lives lost in World War II.
While World War II was the deadliest global conflict, it was not the most lethal in US history – the death toll in the country’s Civil War was over 600,000. But 150 years later, the horrific scale of death in that war is now being overtaken by the number of US fatalities in the pandemic.
“(This is) the worst pandemic in a century,” the President told the US Congress in his first address to a joint session in late April. “The worst economic crisis since the Great Depression. The worst attack on our democracy since the Civil War. Now, after just 100 days, I can report to the nation (that) America is on the move again.”
Vaccination
On being sworn into office on 20 January, President Biden pledged that his administration would deliver 100 million doses of Covid-19 vaccines during his first 100 days.
When he met that pledge on day 58, he raised the target to 200 million doses, reaching that goal on day 92 of his presidency.
The Centres for Disease Control and Prevention Covid tracker showed that on the 100th day of his presidency, 272,030,795 vaccine doses had been delivered to Americans.
President Biden also announced new Covid milestones such as the purchase of an additional 200 million doses from Pfizer and Moderna, the emergency use authorisation for the one-shot Johnson & Johnson (J&J) vaccine and a partnership between J&J and rival company Merck to speed-up manufacturing.
He assembled a coronavirus taskforce on his first day in office to focus on addressing health equity. “The Covid-19 pandemic exposed and exacerbated severe and pervasive health and social inequities in America,” outlined a White House statement when the taskforce was launched.
“It is impossible to change the course of the pandemic without tackling it in the hardest hit communities. In order to identify and eliminate health and social inequities resulting in disproportionately higher rates of exposure, illness, and death, (the President) is directing a Government-wide effort to address health equity.”
On the same day President Biden issued an executive order on a sustainable public health supply chain. According to the White House, its aim was “to secure supplies necessary for responding to the pandemic, so that those supplies are available, and remain available, to the federal government and state, local, tribal, and territorial authorities, as well as to America’s healthcare workers, health systems, and patients”.
He then pushed for immediate coronavirus legislation on a Covid relief package. This $1.9 trillion relief Bill, which was subsequently passed in Congress, facilitated money for direct payments to support Americans hit by the effects of the pandemic, enhanced unemployment benefits, and rental assistance. He also released a vaccine distribution plan under which most Americans have received at least one dose and a large percentage are now fully vaccinated.
On day two of his presidency, President Biden declared that America was rejoining the World Health Organisation (WHO), which overturned Trump’s decision to withdraw from the organisation. He also appointed Dr Anthony Fauci, Director of the National Institute of Allergy and Infectious Disease, to represent the US on the WHO’s executive committee.
Dr Fauci said in a subsequent address to the WHO that

the Biden administration would now back the COVAX project “and support the ACT-Accelerator to advance multilateral efforts for Covid-19 vaccine, therapeutic, and diagnostic distribution, equitable access, and research and development”.
He said the US was also committed to working with partners “to develop new international financing mechanisms for health security and seek an improved, shared system for early warning and rapid response to emerging biological threats”.
The new administration’s delivery of the Covid vaccines programme and the subsequent €1.9 trillion (€1.6 trillion) Covid-19 relief package were widely seen as the major achievements for the President during his first 100 days.
Approval rating
An NBC poll showed 81 per cent of independents approved of how President Biden was handling the pandemic. Polls have also shown that the public strongly supported the Covid-19 relief package.

A majority – 61 per cent – believed that the worst of the pandemic was behind the US. Only 19 per cent believed the worst was yet to come. That was a reversal from an NBC/Wall Street Journal poll before President Biden’s election. In that poll 55 per cent of voters said the worst was yet to come and only 25 per cent said the worst was behind the country.
President Biden’s Vice President Kamala Harris marked her 100th day in office with a visit to a mass vaccination site in Baltimore, where she also touted the administration’s achievements against the pandemic.
Historically, Americans have looked at the first 100 days of an administration as a benchmark to gauge the progress of its priorities. The 100-days yardstick for White House occupants began with the presidency of Franklin D Roosevelt, who took office in 1933.
President Roosevelt’s swift action in the first months of his term to combat the Great Depression made his administration a standard against which Presidents have subse-
THE MEDICAL INDEPENDENT | 31 MAY 2021 12
News Feature
Dr Anthony Fauci
The Covid-19 pandemic exposed and exacerbated severe and pervasive health and social inequities in America
President Joe Biden
quently been measured. In a radio address, President Roosevelt used the phrase “first 100 days” and it stuck. His productivity over this period translated into enormous popularity.
The next President with the highest approval rating after his first 100 days was President John F Kennedy at 83 per cent. The lowest rating was 40 per cent for President Trump.
President Biden’s rating stands at 53 per cent, but he achieves his highest mark (69 per cent) on overall handling of the pandemic.
But the question now is whether President Biden’s success in the battle against Covid-19 will win the “war” and boost his chances of passing other healthcare priorities on his agenda, including a €2.3 trillion infrastructure package and measures to improve access to affordable health insurance and quality healthcare.
American Families Plan
The administration’s American Families Plan has become the President’s vehicle for enhanced healthcare measures and tackling hunger, which has reached appallingly high levels in America.
“The American Families Plan is an investment in our children and our families,” the White House said in a media briefing in April. “It will help families cover the basic expenses that so many struggle with now, lowering health insurance premiums, and continuing the historic reductions in child poverty.”
It will also create a national comprehensive paid family and medical leave programme which the White House says will bring the American system in line with competitor nations.
“The system will also allow people to manage their health and the health of their families and it will provide critical nutrition assistance to families who need it most and expand access to healthy meals to our nation’s students – dramatically reducing childhood hunger.”
Hunger has been a growing problem in the wealthiest nation in the world and has been exacerbated by Covid-19. In 2019, a US Department of Agriculture report on household food insecurity found more than 35 million Americans experienced hunger. Now, due to the pandemic, some 42 million people may experience food insecurity, including 13 million children.
“Hunger can affect people from all walks of life. Many Americans are one job loss or medical crisis away from food insecurity – but some people, including children and seniors, may be at greater risk of hunger than others,” according to advocacy group Feeding America.
Citing a recent study that found US children are getting their healthiest meals at school, the White House said the President’s plan will invest $45 billion (€37 billion) to expand the school meals programmes and increase access to such programmes. President Biden is also planning to build on President Barack Obama’s Affordable Care Act a decade ago, which covers access to affordable health insurance and lower prescription drug costs.
But these plans come with a hefty price tag. The American Families Plan will call for $1 trillion (€828 billion) in new spending and $800 billion (€662 billion) in new tax credits and will aim to significantly expand access to preschool and community college, as well as childcare and healthcare benefits. The plan was rolled out less than a month after President Biden unveiled the $2.3 trillion (€1.9 trillion) infrastructure proposal. This overall agenda adds up to $4 trillion (€3.3 trillion) in spending and that is on top of the already passed $1.9 trillion (€1.6 trillion) Covid relief bill.
The administration says its proposals will be funded by increasing taxes on the assets of wealthier Americans, rather than through hiking taxes of workers wages. “It will ensure that high-income Americans pay the tax they owe under the law – ending the unfair system of enforcement that collects almost all taxes due on wages, while reg-
ularly collecting a smaller share of business and capital income. Importantly, these reforms will also rein in the ways that the tax code widens racial disparities in income and wealth.”
The US Congress has poured tens of billions of dollars into state and local public health departments in response to the pandemic, paying for masks, contact tracers and education campaigns to persuade people to get vaccinated.
But opposition Republicans are wary of such social spending, even though critics note that the party had few qualms about passing major tax breaks aimed mainly at the very wealthy and corporations during the Trump presidency. At present, Democrats have a slim majority in the Senate and the House of Representatives, which they could easily lose in midterm elections in November 2022.

Unless President Biden can get his relief packages passed within the next 18 months, he could face major problems in Congress and even suffer a fate similar to that of President Obama, who found his plans on a range of issues blocked when Republicans won congressional majorities in the 2018 mid-term elections.
Vaccine hesitancy
By all accounts, President Biden is a man in a hurry and eager to seize on the successes of his first 100 day to propel the rest of his agenda forward.
His economists have advised him that a vaccinated public, together with federal stimulus infusions, could trigger a major economic rebound. Perhaps that will happen, but the President is not there yet. Indeed, for all his achievements, the battle against the pandemic has now reached an obstacle on the road – “vaccine hesitancy”. This issue is becoming a major problem for the administration, especially with more contagious variants of Covid-19 threatening the country.
things,” Governor Phil Murphy said at a press briefing.
In Connecticut, Governor Ned Lamont said vaccinated individuals would be able to get a free drink at certain restaurants.

On Alaska’s Kenai Peninsula, medical personnel are bringing the vaccine to any house or business with more than three people. New Orleans partnered with a bar in a "shots for shots" promotion. North Dakota officials are piloting pop-up clinics at Wal-Mart. And states like Georgia, Mississippi, and Montana are worrying about what to do about surplus vaccine as they face more open slots than ever before.
Indeed, a report from the federal health department's assistant secretary for planning and evaluation found that more than 25 per cent of people across Mississippi, Montana, North Dakota, and Wyoming expressed reservations about being vaccinated.
Several states are now administering fewer than 75 per cent of the doses they receive, according to the CDC. Roughly 16 per cent of all American adults are hesitant to get a coronavirus vaccine, says a Census Bureau report.
The problem goes beyond the conspiracy theorists and anti-vaccine promotors. Some people are worried about side-effects and safety, while others may not consider Covid-19 as that much of a problem.
State officials said the focus should now be on meeting people where they are. In North Dakota, the state is partnering with employers to bring the vaccine to employees at workplaces.
Senator Jon Tester of Montana told the Politico news site that “trusted members of the community will have to play a leading role in winning over the unvaccinated”. He also wants the CDC to more explicitly link vaccines to a return to normal life. Their strategy "needs to evolve”, he said. “It needs to be based on science, but it also needs to be based on common sense.”
State governors say they need more help from the Biden administration to reach people who are hesitant. For its part, the administration touts $3 billion (€2.4 billion) already invested in addressing vaccine hesitancy and its new volunteer corps charged with helping to boost vaccine confidence. The all-volunteer group is supposed to amplify the government’s vaccine messaging within their own communities and try to combat scepticism and misinformation.
The administration provided enough vaccines for everyone as well as sufficient vaccinators and vaccination sites. But now many Americans are reluctant to get the vaccination to such an extent that many are being offered inducements like cash or time off work. Indeed, President Biden encouraged all employers to give employees paid time off to get the vaccine and for recovery from any side-effects. He also said he was encouraged by some employers who were giving employees incentives to get vaccinated.
State employees who get vaccinated in Maryland, for example, are eligible for a $100 payment, the state’s Republican governor announced in early May. “Incentives like this are another way to reinforce the importance of getting vaccinated, and we strongly encourage businesses across the state to consider offering incentives to their workers as well,” Governor Larry Hogan said in a statement. “These vaccines are safe and effective, they’re free, and they’re readily available with or without an appointment.”
The City of Detroit introduced a $50 (€41) pre-paid debit card for “good neighbours” each time they brought someone to a vaccine site for an injection. “We’d love to see people embrace the card as an incentive to reach out to friends and family in Detroit to let them know that if they want to get vaccinated, they can be transported by someone they know and trust,” said Mayor Mike Duggan.
In an incentive programme in New Jersey, dubbed “shot and a beer”, those aged 21 and over who got at least one dose in May were eligible for a free beer if they showed their vaccination card at one of several participating breweries. “We’re not going to be afraid to try new
Politics is also seen as a contributing factor in some cases of vaccine hesitancy. A Kaiser Family Foundation survey found nearly one-third of people who identify as Republican (29 per cent) said they would “definitely not” get vaccinated.
However, vaccine hesitancy does not always fit in to a political pattern. Republican-leaning Iowa, for example, is among the top 15 US vaccinated states. The CDC also says poverty may also be a factor. Many counties with high levels of vaccine hesitancy, particularly in the south, are considered by the CDC to be highly socially vulnerable, based on factors like poverty, lack of access to transportation and crowded housing.
Herd immunity
Dr Fauci has suggested that herd immunity may not be reached in the US. “People were getting confused and thinking you’re never going to get the infections down until you reach this mystical level of herd immunity, whatever that number is,” he told The New York Times. “That’s why we stopped using herd immunity in the classic sense. I’m saying: Forget that for a second. You vaccinate enough people, the infections are going to go down.”
On the other hand, a CNN analysis of government vaccine data has suggested the country could reach herd immunity later this summer. In the meantime, the stakes could not be higher for President Biden. While he has notched up impressive results in battling the pandemic in his first 100 days, that may turn out to have been the easy part.
THE MEDICAL INDEPENDENT | 31 MAY 2021 13 Feature News
Hunger can affect people from all walks of life. Many Americans are one job loss or medical crisis away from food insecurity
Governor Ned Lamont
President Franklin D Roosevelt
FMT in the Covid-19 era
Faecal microbiota transplant (FMT) services internationally were severely impacted by Covid-19. David Lynch examines the effect of the pandemic on developing this area in Ireland
In January 2020 the Medical Independent (MI) reported that Stool Bank Ireland aimed to start providing faecal microbiota transplants (FMTs) in Irish hospitals to treat refractory or recurrent Clostridioides difficile infection (CDI) “within a year” of receiving funding.
Stool Bank Ireland is a team of clinicians and researchers from Beaumont Hospital, Dublin; RCSI; Cork University Hospital; and APC Microbiome Ireland, who came together to investigate the feasibility of establishing FMT in Ireland. Early last year, it was announced that the group would receive funding under the Sláintecare Integration Fund.
However, only a few weeks later, the first case of Covid-19 was notified in Ireland and the Irish health service was plunged into the challenge of the pandemic.
The impact of the crisis on non-Covid areas of the heath service has been widely reported. FMT, both in Ireland and internationally, has had to deal with some specific challenges, which have had a serious knock-on effect on the development of the treatment in the area.
‘Potentially life-saving’ FMT involves the transfer of stool from a healthy donor into the patient’s gastrointestinal tract for the purpose of treating recurrent CDI. According to a recent article in The Lancet Microbe, FMT "is a potentially life-saving treatment for patients with refractory and recurrent Clostridioides difficile infection with cure rates of 90 per cent".
In 2018, there were 2,053 notifications of CDI in Ireland, up from 1,763 in 2017, according to figures from the Health Protection Surveillance Centre (HPSC).
"We received funding from Sláintecare to establish Stool Bank Ireland and provide access to FMT for patients in Ireland. Initially the grant was to streamline a supply of FMT from overseas," Dr Fidelma Fitzpatrick, Consultant/Senior Lecturer in Microbiology at the RCSI and member of Stool Bank Ireland, told MI
"Following difficulties encountered sourcing a supply... it became apparent over the course of the grant that it was not possible to source FMT from overseas and that we would need to produce FMT in Ireland."
The Covid-19 pandemic "most certainly impacted the project", confirmed Dr Fitzpatrick, but these difficulties were not confined to this island. "It also impacted FMT and stool banks worldwide," she added.
"Many of us that were clinical based spent most of the first wave working on Covid-19 in our respective hospitals. In addition, as SARS-CoV-2 PCR was reported from faeces in some patients, it was clear that donors and the associated project had to be screened for SARS-CoV-2, so that paused FMT worldwide for a number of months last year."
The scale of the international challenge was highlighted in a February 2021 paper in The Lancet Microbe entitled, 'The journey towards safely restarting faecal microbiota transplantation services in the UK during the Covid-19 era’.
The authors of the paper noted that the "substantial implications with regard to the safe provision of FMT were made evident with emerging reports of detectable SARSCoV-2 virus in stool even before the declaration of the Covid-19 pandemic".
"Furthermore, data suggesting prolonged and potentially infectious faecal virus shedding in stool samples in asymptomatic carriers who tested negative on nasopharyngeal swabs meant FMT donor screening protocols could no longer rely on symptoms and a nasopharyngeal swab alone."
However, the paper outlined a number of protocols intro-
duced in recent months, which have improved the situation and provision of FMT services.
Irish committee
Despite the difficulties posed by Covid, progress was still made on the Irish project last year, with the establishment of a clinical and scientific stool bank committee to provide oversight of the health and safety standards required for FMT and facilitate a nationally coordinated approach. Representatives from gastroenterology, clinical microbiology, infectious diseases, surgery, scientific microbiome research, the Irish Blood Transfusion Service, pharmacy, nursing, patient representation, and external international expert advice from an established Dutch stool bank, came together and formed a steering committee.
"We initially looked at possibilities to import product from abroad once the various international stool banks had appropriate SARS-CoV-2 screening programmes in place," said Dr Fitzpatrick.
"Though we evaluated several potential sources within the
EU they are not set up to export product to another EU country, though several countries had their own stool banks for domestic use.
"We also evaluated the UK as a potential source, however this is also not possible either due to EU data protection compliance issues or supply issues. Though many of us in the past had imported product from the US, for some time now importation from the US is also not possible due to EU data protection compliance issues."

That left the committee investigating whether it could produce product in Ireland.
In January 2017, MI first reported that the Health Products Regulatory Authority (HPRA) was considering the regulation of FMT and planned to issue guidance. In late 2018, this newspaper reported that the HPRA decided to classify FMT as a medicinal product and said it would accept applications for clinical trials using FMT as an investigational medicinal product. However, FMT classification is far from universal internationally and "the regulation of FMT is challenging" on a global level, said Dr Fitzpatrick.

"Stool products do not clearly fit into an existing regulatory framework and there are a number of ways FMT can be classified."
A variety of approaches have been taken in classifying FMT in Europe and around the world, leading to "inconsistencies" in how FMT is regulated, she added.
The approach to FMT regulation by the HPRA is similar to that of regulatory bodies in France, Germany, Switzerland, and the UK. However, in the US, FMT is regulated by the Food and Drugs Administration (FDA) as a biological investigational product. The Australian Therapeutic Goods Administration has recently formulated a regulatory framework for FMT as a biological product.
"In the last few months of the project and after several meetings with the HPRA, we planned to start the process of setting up a GMP [good manufacturing practice] facility in Cork to produce FMT for national use," according to Dr Fitzpatrick.
"This process would have extended beyond the lifetime of the grant, so we sought to lay the groundwork for this under the Sláintecare grant.
"However, it was not possible to do this under the grant, hence we had to close the project on 31 March 2021."
With the closure of the project, and lack of further funding, it was not possible to set up a GMP facility in Cork to produce FMT for national use. Currently, it is not possible to source or produce FMT product for patients with recurrent CDI in Ireland.
"We were advised by the Department of Health via Pobal to prepare a business case to outline what would be required to establish and maintain a stool bank in Ireland. This is currently in preparation."
Building a FMT registry
Despite setbacks, some work was undertaken over the last year to build a faecal microbiota transplant (FMT) registry at a national level.

Ethics submissions to all major public hospitals with a gastroenterology department where FMT would be performed had been drafted to establish a national registry of all FMT recipients.
"Data collected would allow identification of shortterm adverse outcomes and to search for long-term safety concerns, such as chronic conditions like irritable bowel syndrome, obesity, and diabetes," said Dr Fidelma Fitzpatrick, RCSI.
"The function of a registry also serves to guarantee
the continued scrutiny of FMT procedures. The major challenge in submission of ethics applications lay in the absence of a national ethics board committee.
"This resulted in separate ethics application and data sharing agreements needing to be made for each individual hospital in the country. Consultant gastroenterologists from all major hospitals in the country were involved in the ethics application process.
"There was overwhelming support for both the establishment of a stool bank service that could provide FMT samples and the establishment of a national registry for FMT recipients."
THE MEDICAL INDEPENDENT | 31 MAY 2021 14
Stool products do not clearly fit into an existing regulatory framework and there are a number of ways FMT can be classified
News Feature DAVID LYNCH david@mindo.ie
Dr Fidelma Fitzpatrick
Preparing for the worst
Acrippling cyberattack on the health service in the middle of a global pandemic is a nightmare scenario within a nightmare scenario.
It is something that would have been almost impossible to imagine a couple of years ago. But it is, unfortunately, yet another dark twist in our ‘post-Covid’ world and all too real.
There are some broad parallels to draw between the cyberattack and Covid-19. Like pandemics, the seriousness and scale of cyberattacks are only grasped by many after one occurs. And like pandemics, experts have long warned about the likelihood of these incidents, both now and in the future.
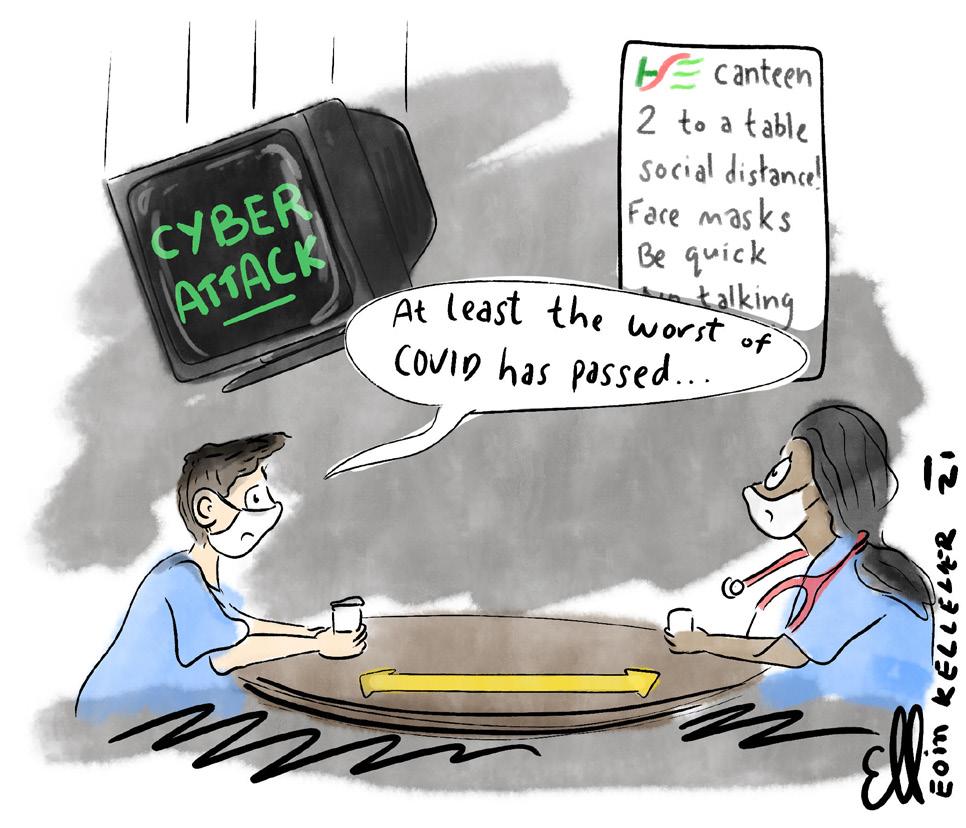
In 2017, the then Minister for Health Simon Harris was questioned about whether there was a strategy to protect the HSE from such events following the global WannaCry ransomware attack in May 2017. This attack targeted computers running the Microsoft Windows operating system by encrypting data and demanding ransom payments in the Bitcoin cryptocurrency.
One of the largest agencies struck by the attack was the NHS in the UK. The HSE was also targeted, but was not as badly affected.
Earlier that year, the Medical Independent reported of concerns expressed by the Master of the Rotunda Hospital, Dublin, Prof Fergal Malone, over the possibility of ransomware attacks.
The “perils of malware software” were cited by Prof Malone in the context of the roll-out of a national electronic health record (EHR) for maternity services, according to hospital board meeting minutes.
And cyberattacks continued. On 13 November 2018, the laboratory information system (LIS) and associated IT infrastructure at the Midland Regional Hospital, Tullamore, suffered a Windows ransomware attack.
Last August, we reported that the HSE or HSE-funded bodies had not
been subject to any ransomware attacks during the Covid-19 pandemic at that point. Experts had warned that healthcare organisations globally were vulnerable to such attacks during the pandemic.
Asked what measures had been taken to ensure IT security with staff working from home, a spokesperson said the HSE used “best in class” products to provide access to systems for remote working.
“All laptops deployed to support remote working users are fully encrypted in accordance with all prevailing HSE security standards,” the spokesperson said at the time.
Following the WannaCry attack, the then HSE Chief Information Officer Mr Richard Corbridge told this newspaper the removal of Microsoft Windows XP from HSE computers and the recruitment of an IT organisation to focus on cyber security were among the possible actions the HSE were contemplating at the time.
Speaking on RTÉ radio after the recent attack, Mr Corbridge said IT upgrades in healthcare were often seen as a cost rather than an investment. In some cases, after the WannaCry attack, the proposed upgrades were deemed too expensive.
Mr Corbridge pointed out that public IT systems, such as those used in healthcare, are targets for cyberattacks, due to under-investment in the area. This is not just an Irish, but an international, issue. He said the attack might been part of a wider probe of health systems across Europe and not specifically targeted against the HSE.
But investment in IT in healthcare in Ireland has fallen short. The slow rollout of the EHR is just one example. Like pandemics, cyberattacks are difficult to prevent due to the increasingly inter-connected world we live in. Both for pandemics and cyberattacks, the first step to ensuring better protection in the future is a recognition of the likelihood of the threat, and that required funding is not just viewed as a cost.
MINDO CARTOON
READER COMMENTS
REACTION TO DR LUCIA GANNON'S COLUMN, 'TEACHING NEW DOGS OLD TRICKS', 20 MAY
"Great article @LuciaGannon." MedisecIreland, @MedisecIreland, 17 May
"Beautifully written. Well done Lucia. New challenges lie ahead."
Dr Martin Daly, @DrMartinDaly, 17 May
"'General practice is a forest that is growing denser year by year'. Love this."
Dr Michelle-Herbert, @MichelleMPS, 17 May
"Those Wednesday day release sessions were invaluable Lucia, I still think of the six hats. Thank you indeed and enjoy the next phase!" Dr Ciara Nolan, @CNiNuall, 17 May
"Best wishes Lucia, Killenaule was my first GP placement in first year of SE scheme! Many fond memories of you, of being at day release, of time in other training practices + great scheme Xmas nights out! What a huge contribution you've made to GP training."
Dr Sinead Fitzpatrick, @SineadNedio78, 17 May
"Very best wishes for the next phase of your career."
Dr Sarah Fitzgibbon, @SarahFitzWiMIN, 17 May
REACTION TO DR PAT HARROLD'S COLUMN, 'HIGH TIME FOR A CHANGE', 20 MAY
"Insightful article on failing approach to drug treatment by a doctor on the frontline."
Dual Diagnosis Ireland, @dualireland, 18 May
REACTION TO DR SARAH FITZGIBBON'S COLUMN, 'GETTING OVER THE STIGMA HURDLE', 10 MAY
"Powerful article, Sarah. Take care of yourself." Jenny Pipe, @jennympipe, 12 May
"Well done @SarahFitzWiMIN, a great piece and so important."
Ag Eisteacht, @AgEisteacht, 11 May
"Really worthwhile piece... you’re a brilliant role model."
Dr Marie Crowley, @MarieCrowley14, 11 May
"Searingly honest. @SarahFitzWiMIN reminding us medics are high risk for stress/ anxiety. Stigma still a potent chilling factor in medicine."
Dr Mike Thompson, @Eastcorkclinsoc, 11 May
"Oh dear! and how wonderful all at the same time ! Well done Sarah."
Dr Carmel Ann Daly, @CarmelanndDaly, 10 May
REACTION TO MS ELAINE NEARY'S ARTICLE, 'HOW DIETITIANS ARE HELPING TO REDUCE GASTROENTEROLOGY WAITING LISTS', 10 MAY
"Fantastic to see how this service improves patient reported symptoms and quality-of-life, while reducing hospital costs and waiting lists." VeronicaMcSharryRD, @VMcSharryRD, 12 May
"A fantastic read by @neary_elaine from Tallaght University Hospital on the role of the specialist gastroenterology dietitian in reducing waiting lists and costs."
Sinead Duggan PhD, @SineadNDuggan, 11 May
Editor: Paul Mulholland paul@mindo.ie
News Editor: Catherine Reilly catherine@mindo.ie
Clinical Editor: Priscilla Lynch priscilla@mindo.ie
Journalists: David Lynch, david@mindo.ie , Niamh Cahill, niamh@mindo.ie, Pat Kelly, pat@greenx.ie
Sub-Editor: Emer Keogh emer@greenx.ie
Designer: Laura Kenny laura@greenx.ie
Advertisements: Graham Cooke graham@greenx.ie
Recruitment: Louis O’Hegarty louis@mindo.ie
Digital Marketing: Emma McInerney emma@greenx.ie
Managing Director: Graham Cooke graham@greenx.ie

facebook.com/MedicalIndependent
twitter.com/med_indonews
If you have anything you would like to share, please email: info@mindo.ie
The Medical Independent is published by GreenCross Publishing Ltd., Top Floor, 111 Rathmines Road Lower, Dublin 6, Ireland Tel: 01 441 0024 Web: www.medicalindependent.ie. GreenCross Publishing (est. 2007) is owned by Graham Cooke (graham@greenx. ie). © Copyright GreenCross Publishing Ltd. 2021. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means –electronic, mechanical or photocopy recording or otherwise – whole or in part, in any form whatsoever for advertising or promotional purposes without prior written permission of the publishers. The Medical Independent endeavours to ensure accuracy of information given and of claims made in articles and advertisements. Nevertheless, no responsibility is accepted in respect of such information or claims. Any opinions expressed by contributors are entirely their own and do not purport to be the views of The Medical Independent
For subscriptions, contact: Daiva Maciunaite, daiva@greenx.ie Ireland €200 incl VAT. UK and International €275.00
THE MEDICAL INDEPENDENT | 31 MAY 2021 Letters to: The Editor, The Medical Independent, Greencross Publishing Ltd, Top Floor, 111 Rathmines Road Lr, Dublin 6 or email paul@mindo.ie Editorial PAUL MULHOLLAND
Thwarting the next global health catastrophe
Would an empowered World Health Organisation help prevent future pandemics?
prevented,” said Johnson Sirleaf. “It is due to a myriad of failures, gaps, and delays in preparedness and response.”
Over 15 months after the Covid-19 pandemic began, the first review of how the world responded has just been published. There will be more local and regional reviews I have no doubt, but for now the World Health Organisation’s (WHO) response is the one under the microscope.
The headline finding is that the WHO should have declared the new coronavirus outbreak in China an international emergency earlier than 30 January 2020. But the panel said the next month was “lost” as countries failed to take strong measures to halt spread of the novel virus. The independent experts called for bold WHO reforms and revitalising national preparedness plans to prevent another “toxic cocktail”.
“It is critical to have an empowered WHO,” panel cochair and former New Zealand Prime Minister Helen Clark told reporters on the launch of the report ‘Covid-19: Make it the last pandemic’.
Co-chair Ellen Johnson Sirleaf, a former President of Liberia, said: “We are calling for a new surveillance and alert system that is based on transparency and allows WHO to publish information immediately.”
The SARS-CoV-2 virus, which emerged in the central Chinese city of Wuhan in late 2019, was allowed to evolve into a “catastrophic” pandemic, the report said.
“The situation we find ourselves in today could have been
Chinese doctors reported cases of unusual pneumonia in December 2019 and informed authorities, while WHO picked up reports from the Taiwan Centres for Disease Control and others, the panel said. But as the coronavirus began spreading across the globe, WHO’s top experts disputed how infectious the virus was, saying it was not as contagious as flu and that people without symptoms only rarely spread the virus. We now know that Sars-CoV-2 transmits even quicker than the flu and that a significant proportion of spread is from people who don’t appear to be sick.
WHO’s emergency committee should have declared an international health emergency at its first meeting on 22 January instead of waiting until 30 January, the report said.
That committee did not recommend travel restrictions due to the limitations of WHO’s international health regulations, which need revamping: “If travel restrictions had been imposed more quickly, more widely, again that would have been a serious inhibition on the rapid transmission of the disease and that remains the same today,” it said.

The review was not entirely negative. It highlighted strengths on which to build a more robust system for the future: Health workers have been stalwart in their efforts. Doctors, nurses, midwives, long-term caregivers, community health workers, and other frontline workers, are still working tirelessly to protect people and save lives. It also said country wealth was not a predictor of success. A number of low- and middle-income countries successfully implemented public health measures, which kept illness and death to a minimum. However, a number of high-income countries did not; vaccines were developed at unprece-
dented speed; and open data and open science collaboration were central to alert and response. The sharing of the genome sequence of the novel coronavirus on an open platform quickly led to the most rapid creation of diagnostic tests in history.
The review also praised the “unstinting” efforts of WHO staff, including our own Dr Mike Ryan.

But the overarching message from the review is that the WHO is underpowered and underfunded and must be reformed to give it the resourcing to be more effective. You can sense a frustration with the traditional WHO approach of diplomacy over frankness, transparency, and accountability. In fact, you can almost smell the fustiness of an organisation that is still firmly embedded in the 1960s from the review.
For example, governments seemed unaware that the emergency declaration was WHO’s “loudest possible alarm” and that it, amazingly, has no authority to declare a pandemic (although it eventually described it that way on 11 March).
Individual countries reacted badly also. Instead of preparing their hospitals for Covid-19 patients, many countries engaged in a “winner takes all” scramble for protective equipment and medicines, the review said. But it did not name these countries.
One of the panels most important recommendations is that a new transparent global system should be set up for probing disease outbreaks, empowering the WHO to deploy investigators at short notice and reveal their findings. And we must hope the Covid-19 catastrophe becomes a rallying cry to ensure governments invest in public health to prevent emergencies and be ready for the inevitable next pandemic.
The dire combination of homelessness and imprisonment
We are more than individuals, but part of a community with shared moral responsibilities
GEORGE WINTER
Read more by George Winter at www.mindo.ie
In ‘Down and Out in Covent Garden’ – from his collected pieces in The Dog’s Last Walk (2017) – Howard Jacobson asserts: “Sentimentality works by our seeing only what we want to see.” This thought was prompted as Jacobson left the Royal Opera House grieving for a victim of operatic tuberculosis, “only to have to pick my way between the homeless camped out in their cardboard boxes….” Jacobson veers from aesthetically pleasing imitation suffering “into misery which no great composer has laboured over and no singer rendered exquisite”. How could such jarring disparities be addressed? Jacobson wasn’t sure.
The philosopher L Susan Stebbing (1885-1943) argued that social change was determined by economics, power and… ideas. The most important of these, Stebbing wrote, was ideas. But which ideas? First, some facts. According to Focus Ireland, there were 8,060 people homeless between 22-28 March 2021 across Ireland, including adults and children. And when Moloney et al investigated ‘Homelessness amongst psychiatric Inpatients: A cross sectional study in the mid-west of Ireland’ they reported in the Irish Journal of Medical Science (15 February 2021) that homelessness can contribute to, and be a result of, mental illness; and “[w]ith homelessness at unprecedented levels, there is a need for the development of tailored programmes aimed at supporting these vulnerable groups”.
And it is the well-chosen word “need” in the previous sen-
tence that is of the utmost importance and which must be used to remind those in government – ie power (see Stebbing’s social change triumvirate above) – of their responsibilities. A need was said dismissively to be “something you want, but are not prepared to pay for”. But as Baroness Mary Warnock pointed out, when she addressed the Royal College of Physicians of Edinburgh on 30 June 1995, “[i]n a perverse way there is truth in this.” If we accept the principle of equality, common goods such as education or a health service generate necessities, “needs,” which are more than “wants”. It is axiomatic that such needs, being matters of entitlement in an equal, civilised society, generate rights. Thus, Moloney et al’s “need for… tailored programmes [to support]… these vulnerable groups” asserts the rights of these vulnerable groups; rights, moreover, which those in government are duty-bound to address. In the context of social change being wrought through economics, power and ideas, the susceptibility of economics and power to err, let’s call it… mishandling, is demonstrated by a quote from Fintan O’Toole’s Ship of Fools: How Stupidity and Corruption Sank the Celtic tiger (2009, p117): “The State ended up subsidising – to the tune of around €2 billion in all – the building of houses whose purpose was to provide shelter, not for real people, but for the taxes of their builders.”
Returning to ideas, on 27 July 2004, in a speech to the Democratic National Convention, Barack Obama said: “If there is a child on the south side of Chicago who can’t read, that matters to me, even if it’s not my child… it is that fundamental belief – I am my brother’s keeper, I am my sister’s keeper – that makes this country work.” Obama’s idea identifies the kernel of a truth that we are more than individuals, but part of a community, a community with shared moral responsibilities. And his choice of words in claiming
that we are our brothers’ and sisters’ keepers is particularly apt, especially in the context of a review from the University of Limerick and Trinity College Dublin recently published in the International Journal of Prisoner Health. In ‘From Nowhere to Nowhere. Homelessness and Incarceration: A Systematic Review and Meta-Analysis’ Bashir et al evaluated 18 studies from the US, Canada, UK, Ireland, and Australia: “The estimated prevalence of initial homelessness was 23.41 per cent and at time of discharge was 29.94 per cent.” The study’s senior author and project supervisor Prof Gautam Gulati of the University of Limerick said: “Homelessness in prisoners is an issue of international concern and one that encompasses fundamental human rights. In Ireland, nearly one-in-six people committed to prison are homeless. These individuals face an uphill struggle in reintegration with society, often with additional challenges, such as severe mental illness.”
For a society that might aspire to be one with shared moral responsibilities, as Obama suggested, the dire combination of homelessness and imprisonment begs the question of whether we have lost our sense of outrage. It is a sign of hope that ideas keep coming. For example, Prof Gulati says, “Every prison must have a specialist in housing and every prison mental health team, a dedicated social worker.” And the Peter McVerry Trust, Ireland’s national housing and homeless charity “has called for an extension to a deadline that applies to a key regulation that allows long-term vacant buildings to be reused as housing”.
A fruitful combination of Stebbing’s vision of social change through economics, power, and ideas can be achieved, but competent politicians committed to honouring the rights of society’s most vulnerable are needed to implement it.
Opinion THE MEDICAL INDEPENDENT | 31 MAY 2021 16
DR MUIRIS HOUSTON
Read more by Dr Muiris Houston at www.mindo.ie @muirishouston
MULTIPLE CHOICE QUESTIONS
Question 1
A viral infection in a COPD patient triggers an exacerbation. Correct medical management of the increased dyspnoea and deep yellow sputum is likely to include

A. Sputum culture.
B. Chest x-ray.
C. Prednisolone 10mg daily for seven days.
D. A course of clarithromycin or amoxicillin.
E. Bronchodilators by nebuliser driven by oxygen.
Immediately after a death grief may result in

A. Feeling of numbness.
B. Disbelief.
C. An urge to cry out loudly.
D. A sense of anger.
E. Awareness of the presence of the deceased.
Question 3
In haemophilia A
A. The disorder is inherited as X-linked recessive.
B. There is a deficiency of factor IX.
C. About 90 per cent of severe cases have presented by the age of four months.
D. Investigation shows a normal platelet count.

E. The partial thromboplastin time is prolonged.
Question 4
Milia
A. Are small epidermoid cysts.
B. Only occur on the face.
C. May itch.
D. May develop in association with sunburn.
E. Can be removed by breaking the surface with a sterile needle.
Question 5
In ankylosing spondylitis
A. Age at onset is as likely to be before as after 40 years.
B. Characteristic symptoms include fatigue.
C. In contrast to mechanical spinal disease, spinal mobility is usually decreased asymmetrically.

D. Peripheral joint involvement occurs at some stage in 20-to-30 per cent of patients.
E. A key management strategy is rest with avoidance of too much exercise.
E. FALSE. Exercise and physiotherapy are of paramount importance.
D. TRUE. Especially in the legs. Some 20 per cent of juvenile-onset cases need hip replacement within 15-to-20 years of disease onset.
C. FALSE. Is decreased symmetrically in both anterior and lateral planes.
B. TRUE. Often more difficult to manage than pain or stiffness.
A. FALSE. Presents in young adulthood and lasts indefinitely.
D. FALSE. No association.
C. FALSE. They are symptomless.
B. FALSE. May occur anywhere, although commonly seen on the face.
A. TRUE. Small white or cream-coloured papules.

ANSWER 4
E. TRUE. But if they are causing no problem no treatment is needed and they frequently disappear spontaneously. ANSWER 5
E. TRUE. In normal person clotting should occur in 25-to35 seconds.
D. TRUE. And without anaemia unless the bleeding has been severe or prolonged.
C. FALSE. By four years - once the children are mobile.
B. FALSE. Factor VIII is deficient. Factor IX is deficient in haemophilia B, which accounts for 15 per cent of congenital haemophilias.
A. TRUE. With a positive family history in 80 per cent of cases.
ANSWER 3
E. TRUE. Common. Attempts to mitigate the sense of loss.
D. TRUE. May be directed at self, family, professionals, or God.
C. TRUE. If unacceptable to family or society, bereaved person attempts to restrain this.
B. TRUE. Common protective reaction.
A. TRUE. Lasts hours or days, but not generally regarded as abnormal unless lasts more than two weeks.
ANSWER 2
E. FALSE. By spacer or nebuliser, but not with oxygen in case the patient has carbon dioxide retention.
D. TRUE. Or doxycycline.
C. FALSE. 30mg daily for seven-to-14 days.
B. FALSE. Only if the patient does not respond to treatment.
FALSE. Would not help unless symptoms have not improved following antibiotic treatment and this has not already been done.
MCQs THE MEDICAL INDEPENDENT | 31 MAY 2021 17
2
1
Question
ANSWER
A.
50mgs once daily
50mgs once daily
Her 10th shopping trip since the day she started BETMIGA1
Prescribing Information: Please read the Summary of Product Characteristics (SPC) before prescribing. Presentation: Prolonged-release tablet, containing mirabegron 25mg/50mg. Indication: Symptomatic treatment of urgency, increased micturition frequency and/or urgency incontinence as may occur in adult patients with overactive bladder (OAB) syndrome. Posology and method of administration: The recommended dose is 50 mg once daily. A lower dose of 25mg is recommended for specific patient populations (renal and hepatic impairment) as well as in specific patient populations in combination with strong CYP3A inhibitors such as itraconazole, ketoconazole, ritonavir and clarithromycin. Renal impairment: End stage renal disease (GFR < 15 mL/min/1.73 m2 or patients requiring haemodialysis): Not recommended. Severe renal impairment (GFR 15 to 29 mL/min/1.73 m2): Reduce dose to 25 mg. Severe renal impairment and concomitant strong CYP3A inhibitors: Not recommended. Moderate renal impairment (GFR 30 to 59 mL/min/1.73 m2): 50 mg. Moderate renal impairment and concomitant strong CYP3A inhibitors: Reduce dose to 25 mg. Mild renal impairment (GFR 60 to 89 mL/min/1.73 m2): 50 mg. Mild renal impairment and concomitant strong CYP3A inhibitors: Reduce dose to 25 mg. Hepatic impairment: Severe hepatic impairment (Child-Pugh Class C): Not recommended. Moderate hepatic impairment
(Child-Pugh B): Reduce dose to 25 mg. Moderate hepatic impairment and concomitant strong CYP3A inhibitors: Not recommended. Mild hepatic impairment (Child-Pugh A): 50 mg. Mild hepatic impairment and concomitant strong CYP3A inhibitors: Reduce dose to 25 mg. The tablet is to be taken once daily, with liquids, swallowed whole and is not to be chewed, divided, or crushed. It may be taken with or without food Contraindications: Hypersensitivity to the active substance or to any of the excipients (see the SPC for a list of excipients). Severe uncontrolled hypertension defined as systolic blood pressure ≥180 mm Hg and/or diastolic blood pressure ≥110 mm Hg. Special warnings and precautions for use: Renal impairment: Betmiga has not been studied in patients with end stage renal disease (GFR < 15 mL/min/1.73 m2 or patients requiring haemodialysis) and, therefore, it is not recommended for use in this patient population. Data are limited in patients with severe renal impairment (GFR 15 to 29 mL/min/1.73 m2); based on a pharmacokinetic study a dose reduction to 25 mg is recommended in this population. This medicinal product is not recommended for use in patients with severe renal impairment (GFR 15 to 29 mL/min/1.73 m2) concomitantly receiving strong CYP3A inhibitors. Hepatic impairment: Betmiga has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and, therefore, it is not recommended for use in this patient population. This medicinal product is not recommended for use in patients with moderate hepatic impairment (Child-Pugh B) concomitantly receiving
strong CYP3A inhibitors. Hypertension: Mirabegron can increase blood pressure. Blood pressure should be measured at baseline and periodically during treatment with mirabegron, especially in hypertensive patients. Data are limited in patients with stage 2 hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg). Patients with congenital or acquired QT prolongation: Betmiga, at therapeutic doses, has not demonstrated clinically relevant QT prolongation in clinical studies. However, since patients with a known history of QT prolongation or patients who are taking medicinal products known to prolong the QT interval were not included in these studies, the effects of mirabegron in these patients is unknown. Caution should be exercised when administering mirabegron in these patients. Patients with bladder outlet obstruction and patients taking antimuscarinic medicinal products for OAB: Urinary retention in patients with bladder outlet obstruction (BOO) and in patients taking antimuscarinic medicinal products for the treatment of OAB has been reported in postmarketing experience in patients taking mirabegron. A controlled clinical safety study in patients with BOO did not demonstrate increased urinary retention in patients treated with Betmiga; however, Betmiga should be administered with caution to patients with clinically significant BOO. Betmiga should also be administered with caution to patients taking antimuscarinic medicinal products for the treatment of OAB. Interactions: Pharmacokinetic interactions: Mirabegron is a substrate for CYP3A4, CYP2D6, butyrylcholinesterase, uridine diphosphoglucuronosyltransferases (UGT), the efflux transporter P-glycoprotein (P-gp) and the influx organic cation transporters (OCT) OCT1, OCT2, and OCT3. Pharmacokinetic interactions involving the potential for other medicinal products to affect mirabegron exposures: Increases in mirabegron exposure due to drug-drug interactions may be associated with increases in pulse rate. Strong CYP3A inhibitors See Posology and administration above for dose adjustments recommended during concomitant use of strong CYP3A inhibitors in patients with renal or hepatic impairment. Mirabegron exposure (AUC) was increased 1.8-fold in the presence of the strong inhibitor of CYP3A/P-gp ketoconazole. CYP2D6 inhibitors: No dose adjustment is needed for mirabegron when administered with CYP2D6 inhibitors (or in patients who are CYP2D6 poor metabolisers). Inducers: Inducers of CYP3A (such as rifampicin) or P-gp may decrease the plasma concentrations of mirabegron. No dose adjustment of mirabegron is required as this effect is not expected to be clinically relevant. Pharmacokinetic interactions involving the potential for mirabegron to affect exposures to other medicinal products: Inhibition of CYP2D6: Moderate and time dependent inhibition of CYP2D6 by mirabegron may result in clinically relevant drug interactions. CYP2D6 activity recovers within 15 days after discontinuation of mirabegron. Caution is advised if mirabegron is co-administered with medicinal
products metabolized by CYP2D6 with a narrow therapeutic index such as thioridazine, Type 1C antiarrhythmics (e.g.flecainide, propafenone) and tricyclic antidepressants (e.g., imipramine, desipramine). Caution is also advised if mirabegron is co-administered with CYP2D6 substrates that are individually dose titrated. Inhibition of P-gp: Mirabegron is a weak inhibitor of P-gp. For patients who are initiating a combination of Betmiga and digoxin, the lowest dose for digoxin should be prescribed initially. Serum digoxin concentrations should be monitored and used for titration of the digoxin dose to obtain the desired clinical effect. The potential for inhibition of P-gp by mirabegron should be considered when Betmiga is combined with sensitive P-gp substrates e.g. dabigatran. Fertility, pregnancy and lactation: The effect of mirabegron on human fertility has not been established. Betmiga is not recommended during pregnancy and in women of child-bearing potential not using contraception. Mirabegron should not be administered during breast feeding. Refer to SPC for full guidance. Driving and use of machines: Betmiga has no or negligible influence on the ability to drive and use machines.
Undesirable effects: Summary of the Safety Profile: the safety of Betmiga was evaluated in 8433 patients with OAB, of which 5648 received at least one dose of mirabegron in the phase 2/3 clinical program, and 622 patients received Betmiga for at least 1 year (365 days). In the three 12-week phase 3 double blind, placebo controlled studies, 88% of the patients completed treatment with this medicinal product, and 4% of the patients discontinued due to adverse events. Most adverse reactions were mild to moderate in severity. The most common adverse reactions reported for patients treated with Betmiga 50 mg during the three 12-week phase 3 double blind, placebo controlled studies are tachycardia and urinary tract infections.
The frequency of tachycardia was 1.2% in patients receiving Betmiga 50 mg.
Tachycardia led to discontinuation in 0.1% patients receiving Betmiga 50 mg. The frequency of urinary tract infections was 2.9% in patients receiving Betmiga 50 mg.
Urinary tract infections led to discontinuation in none of the patients receiving Betmiga 50 mg. Serious adverse reactions included atrial fibrillation (0.2%). Adverse reactions observed during the 1-year (long term) active controlled (muscarinic antagonist) study were similar in type and severity to those observed in the three 12-week phase 3 double blind, placebo controlled studies. The following adverse reactions were observed with mirabegron in the three 12-week phase 3 double blind, placebo controlled studies. The frequency of adverse reactions is defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be established from the available data) Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. The adverse events
are grouped by MedDRA system organ class. Infections and infestations: Common: urinary tract infection Uncommon: vaginal infection, cystitis Psychiatric disorders: Not known: Insomnia*, confusional state* Nervous system disorders: Common: headache* dizziness* Eye disorders: Rare: eyelid oedema Cardiac disorders:
Common: tachycardia Uncommon: palpitation, atrial fibrillation Vascular disorders:
Very rare: Hypertensive crisis* Gastrointestinal disorders: Common: nausea*, constipation*, diarrhoea* Uncommon: dyspepsia, gastritis Rare: lip oedema Skin and subcutaneous tissue disorders: Uncommon: urticaria, rash, rash macular, rash papular, pruritus Rare: leukocytoclastic vasculitis, purpura, angioedema* Musculoskeletal and connective tissue disorders: Uncommon: joint swelling Renal and urinary disorders:
Rare: urinary retention* Reproductive system and breast disorders: Uncommon: vulvovaginal pruritus Investigations Uncommon: blood pressure increased, GGT increased, AST increased, ALT increased (*observed during post-marketing experience) Reporting of suspected adverse reactions: see below. Legal category: POM (S1B) Marketing Authorisation number: EU/1/12/809/003 25mg EU/1/12/809/010 50mg. Marketing
Authorisation holder: Astellas Pharma Europe B.V. Sylviusweg 62, 2333 BE Leiden, The Netherlands. Further information is available from: Astellas Pharma Co., Ltd, 5 Waterside, Citywest Business Campus, Dublin 24. Phone: +3531 467 1555. Summary of Product Characteristics with full prescribing information available upon request. Job number: BET_2019_0002_IE Date of preparation of API: 27 May 2019.
Reporting of suspected adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via:
HPRA Pharmacovigilance Astellas Pharma Co. Ltd
Earlsfort Terrace, IRL - Dublin 2 Tel: + 353 1 467 1555

Tel: +353 1 6764971

Fax: +353 1 6762517
Website: www.hpra.ie E-mail: medsafety@hpra.ie.
E-mail: Irishdrugsafety@astellas.com
Date of preparation: June 2019 References: 1. Freeman R, et al. Current Medical Research and Opinion 2017. https://doi.org/10.1080/03007995.2017.1419170. Approval code: BET_2019_0004_IE
10988_Betmiga_SHOPPING_A4_MAY19_01.indd 1 10/07/2019 16:41
DR EOIN MACCRAITH1 and DR NIALL DAVIS1,2
Cystitis: Risk factors, aetiology, and management in females
Cystitis is a common presentation in women, which can usually be diagnosed and managed in general practice, but onward referral to a urologist is sometimes necessary
Cystitis is defined as an infection and/or inflammation of the bladder. The presentation may involve frequent voiding of small volumes of urine, dysuria, urgency, odorous urine, suprapubic pain, haematuria, urinary incontinence or fever. Cystitis in the presence of infection may be classified as a urinary tract infection (UTI). An uncomplicated UTI is one occurring in a structurally and functionally normal urinary tract. This type of UTI accounts for the majority of cystitis cases in female patients and will typically resolve with a short course of antibiotics. Cystitis may also occur in the absence of a UTI. There are three main causes for non-infectious cystitis. It may occur after pelvic radiotherapy and is often referred to as radiation cystitis. It may also occur if the patient has a history of cyclophosphamide or ketamine use. Interstitial cystitis (or more recently known as bladder pain syndrome) is a chronic debilitating disorder with similar symptoms to infectious cystitis, but is diagnosed by excluding all other causes.
Risk factors and aetiology
Risk factors for cystitis in females include; increasing age, reduced oestrogen (menopause), previous UTI, diabetes mellitus, pregnancy, institutionalised elderly patients, stone disease, indwelling catheters, voiding dysfunction and genitourinary tract malformation. Risk factors in pre-menopausal patients include sexual intercourse, use of spermicide, new sexual partner, history of childhood UTI, and mother with a history of UTI. The most common causes of cystitis are Escherichia coli (E. coli), a Gram-negative bacillus, which accounts for 85 per cent of community-acquired and 50 per cent of hospital-acquired cystitis infections. Other common causative organisms include Staphylococcus saprophyticus, Proteus mirabilis, and Klebsiella. Bacteria from the bowel colonise the perineum, vagina, and distal urethra. From here the bacteria ascend along the urethra and reach the bladder causing cystitis.
Investigations
The first investigation for cystitis should be a urine dipstick, which has several components to interpret. Leukocyte esterase activity detects the presence of white blood cells (WBCs) in the urine. The presence of WBCs in the urine is referred to as pyuria and implies an inflammatory response of the urothelium (lining of the bladder) to a bacterial infection. This component of the urine dipstick has a sensitivity of 75-to-95 per cent for detection of infection. The next component is nitrites. These are not normally found in the urine and their presence suggests there is bacteria in the urine (bacteriuria). The sensitivity of this test is only 35-to-85 per cent (false negatives are common), but if nitrites are positive on a dipstick then it is
CASE STUDY
A 53-year old female presents to her GP with a three-day history of urinary frequency, dysuria and malaise. She has been treated for two previous UTIs this year at a different practice. She had no previous history of UTIs before this year. Her urine dipstick is positive for nitrites only. She is managed with a five day course of trimethoprim 200mg PO BD and her symptoms fully resolve. The MSU sample later showed E. coli, which is sensitive to trimethoprim and nitrofurantoin. An outpatient referral is sent to a urologist because she is now classified as suffering from recurrent UTIs.
The urologist organised an ultrasound kidneys and bladder which is unremarkable and shows that the bladder empties to completion. On examination the urologist noted some early evidence of atrophic vaginitis related to lack of oestrogen. The patient is commenced on local oestrogen therapy in the form of Vagifem.
highly likely there is an infection because the specificity is very high (>90 per cent). A mid-stream urine (MSU) sample, which is collected for a dipstick test, may also be sent to a lab for microscopy and culture. False positives on microscopy may occur due to vaginal commensal bacteria, such as lactobacillus and Corynebacterium. To make a diagnosis of an uncomplicated UTI it is recommended that a urine culture would demonstrate >103 colony forming units per millilitre (cfu/mL).
Usually a solitary episode of cystitis does not require any further investigations. However, further tests such as renal ultrasound, cystoscopy or CTKUB (kidney, ureter and bladder) may be necessary in certain clinical scenarios such as recurrent UTIs, unusual organisms on MSU, pregnancy or symptoms of pyelonephritis. Ultrasound may detect hydronephrosis, renal calculi, or structural abnormalities, and can be used to calculate the post-void residual volume in the bladder.
CTKUB may detect renal, ureteric or bladder calculi. Cystoscopy may detect rare causes of recurrent UTIs such as urethral stenosis, bladder cancer, bladder calculi or a fistula.
Management
Acute cystitis episode
For acute uncomplicated cystitis, treatment with one of the following regimens is recommended: Nitrofurantoin 50mg PO QDS for five days, trimethoprim 200mg PO BD for five days, or fosfomycin 3g PO single dose for one day. Acute cystitis episodes may also be prevented with general measures such as good fluid intake, avoiding con-
At a 12-month follow up appointment with her urologist the patient reports an initial good response to the treatment for eight months, but in the last four months she has had three UTIs. Two of these episodes were associated with visible haematuria, and she is an ex-smoker. She undergoes a cystoscopy and CT urogram; both of which are unremarkable. The MSU on each occasion grows E. coli, which is sensitive to trimethoprim and nitrofurantoin. The patient is commenced on low-dose continuous antibiotic prophylaxis in the form of trimethoprim 100mg PO nocte for six months as well as a probiotic in the form of Udo's Choice Super 8. She continuous to use Vagifem at the lowest effective dose as she found this to be beneficial. At a 12-month follow up appointment she is well and reports no UTIs for one year. She has been finished her low-dose antibiotic prophylaxis for six months and is maintained on probiotics and local oestrogen therapy.
stipation, cranberry juice, double voiding and wiping from front to back after voiding.
Recurrent UTIs
Recurrent UTIs are defined as >two infections in six months or three within 12 months. For recurrent UTIs, the European Association of Urology (EAU) guidelines panel recommends offering either three-to-six months of continuous lowdose antibiotic prophylaxis or post-coital antibiotic prophylaxis (single dose only). Continuous low-dose regimens include nitrofurantoin 50-to-100mg PO nocte, trimethoprim 100mg PO nocte, or fosfomycin 3g every 10 days. Patients with good compliance should be offered self-diagnosis and self-treatment with a short-course regimen of antibiotics (the same regimens we discussed for acute cystitis episodes) for recurrent UTIs. It is important to avoid using unnecessarily long courses (>five days) of antibiotics for acute cystitis episodes in this group of patients in order to prevent reduced efficacy of antibiotics.
There are a number of treatment measures for UTIs besides antibiotic prophylaxis – effective lifestyle modification, particularly while at work, including avoiding habitual delayed urination and maintaining good fluid intake. It is important to avoid the spermicides that are present on condoms and the diaphragm.
Post-menopausal women have a lack of oestrogen resulting in loss of protective lactobacilli. Topical oestrogen replacement with products such as Ovestin or
Vagifem, but not oral oestrogen, has been shown to be effective at reducing recurrent UTIs.
Proanthocyanidin (PAC) contained in cranberry products block bacteria adhering to the lining of the urinary tract and consumption of cranberry products has been shown to reduce the absolute risk of UTIs by 20 per cent.
Applying natural yoghurt to the vagina may help to restore the natural protective flora.
Probiotics (lactobacillus) are generally regarded as effective at reducing recurrent episodes of cystitis, however, studies have failed to demonstrate this benefit. Udo's Choice Super 8 is an example of a probiotic that is commonly recommended by urologists and is available without prescription.
D-Mannose is a product which can also be purchased without prescription and it has been shown that taking 2g of the powder dissolved in 200ml water daily reduces the risk of UTIs by 45 per cent. D-Mannose is thought to work in a similar manner to cranberry products by blocking the binding of bacteria to the urothelium.
In diabetic patients with recurrent cystitis it is important to improve glucose control to reduce the frequency of episodes. Courses of Hiprex 1g PO OD have been shown to reduce UTI episodes, but it is not commonly used.
The efficacy of acupuncture has been investigated for recurrent UTIs, and in a small series it was shown to be more effective than placebo, but there is not strong enough evidence to recommend it as a treatment. Administration of hyaluronic acid (iAluril) into the bladder once per week for four weeks has been investigated for recurrent cystitis. A small series showed an 86 per cent reduction in UTI episodes. This treatment option appears from small studies to be effective, but is costly to administer in terms of healthcare resources.
Conclusion
In summary, cystitis is a common condition in females which can be diagnosed with clinical history, examination and midstream urine collection. It is typically caused by E. coli from the bowel which colonises the perineum and vagina, and ascends the urethra to cause cystitis. Acute episodes should be treated with appropriate short-course antibiotics and patients given information on general preventative measures. No further investigations are required in one-off uncomplicated UTI episodes. Recurrent UTIs warrant referral to a urologist for further investigation, which may include imaging and cystoscopy to exclude structural or functional urinary tract abnormalities or underlying bladder pathology. Management of recurrent cystitis varies by age, but includes continuous low-dose prophylactic antibiotics, post-coital antibiotic prophylaxis, lifestyle modifications, topical oestrogen, cranberry products, probiotics, and D-Mannose.
References on request
THE MEDICAL INDEPENDENT | 31 MAY 2021 19 Urology Clinical
1 StAR MD Research Fellow, Blackrock Clinic and RCSI, Dublin. 2 Consultant Urologist and Senior Lecturer, Beaumont Hospital and RCSI, Dublin
PROF JAMES MALONE-LEE, Emeritus Professor of Medicine, University College London (UCL)
Confronting the urinalysis tyrant
HOW OFTEN do you meet a patient who describes symptoms of urinary tract infection (UTI), but the urinalyses, whether dipsticks or culture, prove negative? Have you noticed the increasing numbers of predominantly women who keep re-presenting with lower urinary tract symptoms (LUTS) that defy the advice of the tests that you use? You are doing right by the guidelines and you have taken note of the contemporary concerns about antimicrobial resistance (AMR). These patients are baffling aren’t they? The specialists write about ‘painful bladder syndrome’ (PBS), renamed ‘bladder pain syndrome’ (BPS) or ‘interstitial cystitis’. So, the patients are ultrasounded, CT and MRI scanned. They are urodynamicked, cystoscoped, bladder-stretched, urethra-dilated, and then instilled with various concoctions, but frustratingly still they return.
This perplexing problem has been my obsession for forty years, and with our happy gang of researchers it would seem that we have come up with some useful answers. Today we have sufficient knowledge for me to write a book about it, Cystitis Unmasked, that is pitched at clinicians and informed patients alike. There is quite a story because our science found that the guidelines and urinalyses methods were incorrect. That is a gentle adjective because the truth is that they are calamitously wrong. However, we have learned that reporting such science to power is a tricky business. We have spent a decade deflecting a string of criticisms by the chaps in charge who seemed outraged by the outcomes of our science. Feelings ran so high that we felt obliged to conduct the studies necessary to address every proposition that was floated to explain away what we were trying to report. Thus, we evaluated one ‘no true Scotsman’ argument after another in a dispiriting sequence of negative experiments. However, today I may report that the original findings have weathered a hugely detailed critical scrutiny, so we have some confidence in what we have published.
The dipstick test1 and the urine culture2,3 are grossly unreliable witnesses of the events extant in the urinary tract. To dismiss the diagnosis of UTI, despite appropriate symptoms, because one or both of these tests are negative, is a serious fallacy, with the technical term of ‘ignoring the base rate’. The scientific truth is that if the patient has symptoms the probability of a UTI is high indeed and a negative test is probably a false negative.4
The normal bladder is not sterile, but hosts a complex polymicrobial microbiome of several hundreds of different species,
and a 95 per cent overlap with patients who have UTI.5 There is no truth in the claim that the microbe isolated by a midstream urine culture (MSU) is the proven causative organism. The problem of causation is unresolved and applies equally to enhanced cultures and genomic methods. We may isolate microbe(s) but we haven’t a clue whether they are friend or foe. If you treat on the sensitivity data obtained from a culture you are more likely to fan the flames of AMR than treat the patient successfully.6,7 Simple, narrow-spectrum, urinary antibiotics in sufficient dose will perform just as well with less AMR. ‘Mixed growth of doubtful significance’ is of considerable significance in reflecting the pathology of UTI.2 The epithelial cells identified in urinalyses are not indicators of contamination, but important biological manifestations of active cystitis.8
It is not just the testing that is in such a parlous state; the treatment guidelines are equally dubious. Three-day and 14day courses of antibiotic for acute UTI carry a 25-to-35 per cent failure rate9 yet the advice is that you should manage such un-
My experience of a misdiagnosed chronic UTI Lynda Spain, Dublin
I'd like to draw your attention to a major calamity regarding testing for UTIs, which is resulting in millions of women (and a few men) being wrongly diagnosed or worse, being told their symptoms are all in their head and then being left to suffer.
To cut a long story short, I have been suffering with urinary tract symptoms for three years and I was bedridden from the pain. Every time my urine was sent off to be analysed it always came back negative. I couldn't understand it. I ended up being diagnosed with interstitial cystitis – which basically means inflamed bladder. My question for three years was "what is the root cause of the inflammation in my bladder?". I was basically told to just learn to live with it. I was offered bladder instillations which didn't work. I was in a very bad way.
Luckily, through social media, I came across Prof James Malone-Lee who has been researching chronic UTI for 40 years and has a clinic in Harley Street, London. I flew over to see him (well, a member of his team) and they took a fresh sample of my urine and immediately looked at it under a microscope and it turned out that I had a raging chronic embedded UTI. Of course, I knew this all
along from my symptoms. I started my treatment of long-term high-dose antibiotics just over a year ago and I have gone from being bedridden to having a relatively normal life. It will be a long road to full recovery as I'm still left with symptoms of frequency. There are thousands (7.4K) of women and a few men who are members of my support group and we are all suffering. We all have our own stories of being dismissed and gaslighted regarding our symptoms.
Some of them are suicidal, they've lost marriages, relationships have broken up, they are housebound, and can no longer work or socialise because they are being denied medication to treat their UTIs. Can you imagine living with a constant UTI?
I know a lot of women who are desperate and are travelling to the UK for treatment, but what we'd love most is for the current guidelines around UTI testing to be abandoned and diagnostic and teatment guidelines revisited to reflect the best available evidence, and to back research to find better treatments and, lastly, that we can be treated in Ireland.
It's time for change.
fortunates using a discredited culture.10
This is all most worrying. The science has been done carefully and additional work has been achieved to address the common arguments that have been used to claim that none of this could be true. The time has come for those who have the relevant influence to insist that the guidelines and regulations should be re-examined in the light of this new knowledge. It is most unfair on the patients to do otherwise.
To conclude, please consider two images. The first is a confocal micrograph (Figure 1) achieved by Dr Harry Horsley PhD, a most gifted researcher in our centre. It shows urothelial cells extracted from a urine sample and reveals microbes adherent to the surfaces of these cells, but others parasitising these unfortunate cells in order to set up a chronic infection that will be hard to eradicate. This is the pathology that explains those patients who keep returning to you with recalcitrant symptoms but negative tests.
The second is the graph (Figure 2) of the NHS returns for admissions for IC/PBS/BPS annually since 1998. The outpatient attendances paint a similar picture. There is a most worrying rise in the incidence. It started in 2002 and that follows the publication of the first guidelines stipulating three-day antibiotic courses for acute UTI. We do not know whether adherence to those restrictions is the cause of this phenomenon, but that science supports such an hypothesis is certainly worth thinking about.
Prof Malone-Lee’s book is Cystitis Unmasked. TFM Publishing Ltd (1 February 2021). His author royalties will be donated to the current research programme at UCL.
This article first appeared in BMJ Life (https://bjgplife. com/2021/03/19/confronting-the-urinalysis-tyrant/) and was reproduced with kind permission from the author and Editor.
References
1. Kupelian AS, Horsley H, Khasriya R, Amussah RT, Badiani R, Courtney AM, et al. Discrediting microscopic pyuria and leucocyte esterase as diagnostic surrogates for infection in patients with lower urinary tract symptoms: Results from a clinical and laboratory evaluation. BJU Int 2013; 112(2): 231-8
2. Sathiananthamoorthy S, Malone-Lee J, Gill K, Tymon A, Nguyen TK, Gurung S, et al. Reassessment of routine midstream culture in diagnosis of urinary tract infection. J Clin Microbiol 2019; 57(3): 01452-18
3. Gill K, Kang R, Sathiananthamoorthy S, Khasriya R, Malone-Lee J. A blinded observational cohort study of the microbiological ecology associated with pyuria and overactive bladder symptoms. Int Urogynecol J 2018; 29(10): 1493-500
4. Khasriya R, Barcella W, De Iorio M, Swamy S, Gill K, Kupelian A, et al. Lower urinary tract symptoms that predict microscopic pyuria. Int Urogynecol J 2018; 29(7): 1019-28
5. Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, et al. Urine is not sterile: Use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014; 52(3): 871-6
6. Swamy S, Barcella W, De Iorio M, Gill K, Khasriya R, Kupelian AS, et al. Recalcitrant chronic bladder pain and recurrent cystitis, but negative urinalysis: What should we do? Int Urogynecol J 2018; 29(7): 1035-43
7. Swamy S, Kupelian AS, Khasriya R, Dharmasena D, Toteva H, Dehpour T, et al. Cross-over data supporting long-term antibiotic treatment in patients with painful lower urinary tract symptoms, pyuria, and negative urinalysis. Int Urogynecol J 2019; 30(3): 409-14
8. Horsley H, Malone-Lee J, Holland D, Tuz M, Hibbert A, Kelsey M, et al. Enterococcus faecalis subverts and invades the host urothelium in patients with chronic urinary tract infection. Plos One 2013; 8(12): e83637
9. Zalmanovici TA, Green H, Paul M, Yaphe J, Leibovici L. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Syst Rev 2010; (10): CD007182
10. NICE. Urinary tract infection (lower) – women. London: National Institute for Health and Care Excellence, 2014. Available from: http:// cks.nice.org.uk/urinary-tract-infection-lower-women#!topicsummary
Clinical Urology THE MEDICAL INDEPENDENT | 31 MAY 2021 20
Figure 1: Urothelial cells extracted from a urine sample
Figure 2: NHS returns for admissions for IC/PBS/BPS
Abbreviated Prescribing Information - Vesomni 6 mg/0.4 mg modified release tablets. Please read the Summary of Product Characteristics (SPC) before prescribing. Presentation: Each tablet contains a layer of 6 mg solifenacin succinate, corresponding to 4.5 mg solifenacin free base and a layer of 0.4 mg tamsulosin hydrochloride, corresponding to 0.37 mg of tamsulosin free base. Indication: Treatment of moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with benign prostatic hyperplasia (BPH) in men who are not adequately responding to treatment with monotherapy. Posology and method of administration: Adultmales,includingolderpeople: One Vesomni tablet (6 mg/0.4 mg) once daily taken orally with or without food. The maximum daily dose is one Vesomni tablet. The tablet must be swallowed whole, intact without biting or chewing. Do not crush the tablet. Special populations (see also contraindications below):
Renal impairment: Severe renal impairment (creatinine clearance ≤ 30 mL/min): Treat with caution, maximum daily dose in these patients is one Vesomni tablet.
Hepatic impairment: Moderate hepatic impairment (Child-Pugh score of 7-9): Treat with caution, maximum daily dose in these patients is one Vesomni tablet. In patients with severe hepatic impairment (Child-Pugh score > 9), the use of Vesomni is contraindicated. Concomitant treatment withmoderateandstronginhibitorsofCYP4503A4: e.g. verapamil, ketoconazole, ritonavir, nelfinavir, itraconazole: Treat with caution, maximum daily dose should be limited to one Vesomni tablet. Paediatricpopulation: There is no relevant indication for use of Vesomni in children and adolescents.
Contraindications: Patients with hypersensitivy to the active substance(s) or to any of the excipients (see SPC). Patients undergoing haemodialysis. Patients with severe hepatic impairment. Patients with severe renal impairment who are also treated with a strong cytochrome P450 (CYP)3A4 inhibitor e.g. ketoconazole. Patients with moderate hepatic impairment who are also treated with a strong CYP3A4 inhibitor e.g. ketoconazole. Patients with severe gastrointestinal conditions (including toxic megacolon), myasthenia gravis or narrow-angle glaucoma and patients at risk for these conditions.
Patients with a history of orthostatic hypotension. Special Warnings and Precautions for Use: Vesomni should be used with caution in patients with: severe renal impairment; risk of urinary retention; gastrointestinal obstructive disorders; risk of decreased gastrointestinal motility; hiatus hernia/ gastroesophageal reflux and/or who are concurrently taking medicinal products (such as bisphosphonates) that can cause or exacerbate oesophagitis; autonomic neuropathy. The patient should be examined in order to exclude the presence of other conditions, which can cause similar symptoms to benign prostatic hyperplasia. Other causes of frequent urination (heart failure or renal disease) should be assessed before treatment with Vesomni is initiated. If a urinary tract infection is present, appropriate antibacterial therapy should be started. QT prolongation and Torsade de Pointes have been observed in patients with risk factors, such as pre-existing long QT syndrome and hypokalaemia, who are treated with solifenacin succinate. Angioedema with airway obstruction has been reported in some patients on solifenacin succinate and tamsulosin. If angioedema occurs, Vesomni should be discontinued and not restarted. Appropriate therapy and/or measures should be taken. Anaphylactic reaction has been reported in some patients treated with solifenacin succinate. In patients who develop anaphylactic reactions, Vesomni should be discontinued and appropriate therapy and/or measures should be taken. As with other alpha1-adrenoceptor antagonists, a reduction in blood pressure can occur in individual cases during treatment with tamsulosin, as a result of which, rarely, syncope can occur. Patients starting treatment with Vesomni should be cautioned to sit or lie down at the first signs of orthostatic hypotension (dizziness, weakness) until the symptoms have disappeared. The ‘Intraoperative Floppy Iris Syndrome’ (IFIS, a variant of small pupil syndrome) has been observed during cataract and glaucoma surgery in some patients on or previously treated with tamsulosin hydrochloride. IFIS may increase the risk of eye complications during and after the operation. Therefore, the initiation of therapy with Vesomni in patients for whom cataract or glaucoma surgery is scheduled is not recommended. Discontinuing treatment with Vesomni 1-2 weeks prior to cataract or glaucoma surgery is anecdotally considered helpful, but the benefit of treatment discontinuation has not been established. During pre-operative assessment, surgeons and ophthalmic teams should consider whether patients scheduled for cataract or glaucoma surgery are being or have been treated with Vesomni in order to ensure that appropriate measures will be in place to manage IFIS during surgery. Vesomni should be used with caution in combination with moderate and strong inhibitors of CYP3A4 and it should not be used in combination with strong inhibitors of CYP3A4, e.g., ketoconazole, in patients who are of the CYP2D6 poor metaboliser phenotype or who are using strong inhibitors of CYP2D6, e.g., paroxetine.
Interactions: Pharmacological interactions: Concomitant medication with other anticholinergic medicinal products may result in more pronounced therapeutic and undesirable effects. Allow approximately one week after stopping treatment with Vesomni before commencing any anticholinergic therapy. The therapeutic effect of solifenacin may be reduced by concomitant administration of cholinergic receptor agonists. Pharmacokinetic interactions: Pharmacokinetic interactions involving the potential for other medicinal products to affect Vesomni exposures: Interactions with CYP3A4 and CYP2D6 inhibitors: See Contraindications, Posology and administration and Special warnings and precautions above. Concomitant administration may lead to increased exposure to both solifenacin (ketoconazole 400 mg/day resulted in a 1.5-fold increase in Cmax and a 2.8-fold increase in AUC). and tamsulosin (ketoconazole 400 mg/day resulted in a 2.2-fold increase in Cmax and a 2.8-fold increase in AUC). Vesomni should be used with caution in combination with strong CYP3A4 inhibitors. Vesomni should not be given together with strong CYP3A4 inhibitors in patients who are also CYP2D6 poor metabolizer phenotype or who are using strong CYP2D6 inhibitors. See SPC for details of the effects of other CYP3A4 and CYP2D6 inhibitors. Inducers: Inducers of CYP3A4 (e.g. rifampicin) may decrease the plasma concentrations of solifenacin and tamsulosin. Information available for the individual active substances: Solifenacin can reduce the effect of medicinal products that stimulate the motility of the gastrointestinal tract, such as metoclopramide and cisapride. Solifenacin did not affect the pharmacokinetics of digoxin, or the pharmacokinetics or effect on prothrombin time of R- or S-warfarin. Co-administration of tamsulosin and other alpha1-adrenoreceptor antagonists could lead to hypotensive effects. Diclofenac and warfarin may increase the elimination rate of tamsulosin. No interactions have been seen when tamsulosin was given concurrently with atenolol, enalapril or theophylline. Fertility, pregnancy and lactation: The effect of Vesomni on fertility has not been established. Ejaculation disorders have been observed in short and long term clinical studies with tamsulosin. Events of ejaculation disorder, retrograde ejaculation and ejaculation failure have been reported in the post authorization phase. Vesomni is not indicated for use in women. Driving and use of machines: No studies have been performed, however patients should be informed about the possible occurrence of dizziness, blurred vision, fatigue and uncommonly somnolence, which may negatively affect the ability to drive or use machines. Undesirable Effects: Summary of the safety profile: Vesomni may cause anticholinergic undesirable effects of, in general, mild to moderate severity. The most frequently reported adverse reactions during the clinical studies performed for the development of Vesomni were dry mouth (9.5%), followed by constipation (3.2%) and dyspepsia (including abdominal pain; 2.4%). Other common undesirable effects are dizziness (including vertigo; 1.4%), vision blurred (1.2%), fatigue (1.2%), and ejaculation disorder (including retrograde ejaculation; 1.5%). Acute urinary retention (0.3%, uncommon) is the most serious adverse drug reaction that has been observed during treatment with Vesomni in clinical studies. List of adverse reactions: the ‘Vesomni frequency’ below reflects adverse drug reactions that have been observed during the double-blind clinical studies performed for the development of Vesomni (based on reports of treatment-related adverse events, which have been reported by at least two patients and occurred with a frequency higher than for placebo in the double-blind studies). The ‘solifenacin frequency’ and ‘tamsulosin frequency’ below reflect adverse drug reactions (ADRs) previously reported with one of the individual components (as presented in the Summary of Product Characteristics (SmPCs) of solifenacin 5 and 10 mg and tamsulosin 0.4 mg respectively that may also occur when receiving Vesomni (some of these have not been observed during the clinical development program of Vesomni). The frequency of adverse reactions is defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data). The adverse events are grouped by MedDRA system organ class preferred term (PT). Vesomni frequency: Nervous system disorders: Common: dizziness Eye disorders: Common: vision blurred Gastrointestinal disorders: Common: dry
Psychiatric disorders: Very rare: hallucination*, confusional state* Not known:
Gastrointestinal disorders: Very common: dry mouth Common: dyspepsia, constipation, nausea, abdominal pain Uncommon: gastro-oesophageal reflux disease, dry throat Rare: vomiting*, colonic obstruction, faecal impaction, Not known: ileus*, abdominal discomfort* Hepatobiliary disorders: Not known: liver disorder*, liver function test abnormal* Skin and subcutaneous tissue disorders: Uncommon: dry skin Rare: pruritus*, rash* Very rare: urticaria*, angioedema*, erythema multiforme* Not known: exfoliative dermatitis* Musculoskeletal and connective tissue disorders: Not known: muscular weakness* Renal and urinary disorders: Uncommon: difficulty in micturition Rare: urinary retention***Not known: renal impairment* General disorders and administration site conditions: Uncommon: fatigue, perhiperal oedema Tamsulosin 0.4mg frequency#: Nervous system disorders: Common: dizziness Uncommon: headache Rare: syncope Eye disorders: Not known: vision blurred*, Intraoperative Floppy Iris Syndrome (IFIS)**, visual impairment* Cardiac disorders: Uncommon: palpitations Not known: atrial fibrillation*, arrhythmia*, tachycardia* Vascular disorders: Uncommon: orthostatic hypotension Respiratory, thoracic and mediastinal disorders: Uncommon: rhinitis Not known: dyspnoea*, epistaxis* Gastrointestinal disorders: Uncommon: constipation, nausea, diarrhea, vomiting Skin and subcutaneous tissue disorders: Uncommon: pruritus, rash, urticaria Rare: angioedema Very Rare: Stevens-Johnson syndrome Not known: erythema multiforme*, exfoliative dermatitis* Reproductive system and breast disorders: Common: ejaculation disorders including retrograde ejaculation and ejaculation failure Veryrare: priapism General disorders and administration site conditions: Uncommon: asthenia. : The ADRs from solifenacin and tamsulosin included are the ADRs listed in the summary of product characteristics of both products. *: from post-marketing reporting. Because these spontaneously reported events are from the worldwide post-marketing experience, the frequency of events and the role of solifenacin or tamsulosin and their causation cannot be reliably determined. **: from post-marketing reporting, observed during cataract and glaucoma surgery. ***: see Special warnings and precautions for use. Long-term safety of Vesomni: The profile of undesirable effects seen with treatment up to 1 year was similar to that observed in the 12-week studies. The product is well-tolerated and no specific adverse reactions have been associated with long-term use. Description of selected adverse reactions: For urinary retention see Special warnings and precautions for use. Older people: The therapeutic indication of Vesomni, moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with BPH, is a disease affecting elderly men. The clinical development of Vesomni has been performed in patients 45 to 91 years of age, with an average age of 65 years. Adverse reactions in the elderly population were similar to the younger population. Reporting of suspected adverse reactions: see below. Overdose: Overdosage with the combination of solifenacin and tamsulosin can potentially result in severe anticholinergic effects plus acute hypotension. Refer to SPC for details of treatment of overdose. Legal Category: Prescription Only Medicine (SIB). Nature and contents of container: Aluminium blister packs containing 30 tablets. Product Authorisation Number: PA1241/016/001. Marketing Authorisation holder: Astellas Pharma Co. Ltd. Further information is available from: Astellas Pharma Co. Ltd, 5 Waterside, Citywest Business Campus, Naas Road, Dublin 24. Phone: +3531 467 1555. Summary of Product Characteristics with full prescribing information available upon request. Job number: VESOM_2019_0001_IE Date of preparation of API: 24 May 2019
Reporting suspected adverse reactions after authorisation of the medicinal product is important.

Renal
disorders: Uncommon:
Common:
Common:
Uncommon: urinary
Immune
disorders: Not
anaphylactic
Metabolism
Not
appetite*, hyperkalemia*
delirium* Nervous system disorders: Uncommon: somnolence, dysgeusia Rare: dizziness*, headache* Eye disorders: Common: vision blurred Uncommon: dry eyes Not known: glaucoma* Cardiac disorders: Not known: palpitations*, Torsade de Pointes*, electrocardiogram QT prolongation*, atrial fibrillation*, tachycardia* Respiratory, thoracic and mediastinal disorders: Uncommon: nasal dryness Not known: dysphonia*
mouth, dyspepsia, constipation Skin and subcutaneous tissue disorders: Uncommon: pruritus
and urinary
Urinary retention*** Reproductive system and breast disorders:
ejaculation disorders including retrograde ejaculation and ejaculation failure General disorders and administration site conditions:
fatigue Solifenacin5mg&10mgfrequency#: Infections and infestations:
tract infection, cystitis
system
known:
reaction*
and nutrition disorders:
known: decreased
It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance Astellas Pharma Co. Ltd Earlsfort Terrace, IRL - Dublin 2 Tel: + 353 1 467 1555 Tel: +353 1 6764971 E-mail: Irishdrugsafety@astellas.com Fax: +353 1 6762517 Website: www.hpra.ie E-mail: medsafety@hpra.ie. 49%
LUTS
2 A combined force against LUTS and BPH References: 1. Sexton CC, et al. BJU Int 2009; 103(Suppl 3): 12-23. 2. VESOMNI Summary of Product Characteristics. Date of preparation: July 2019 Job code: VESOM_2019_0003_IE
of men with
report bladder and prostate symptoms1 VESOMNI treats the symptoms of both the bladder and prostate
THERESA LOWRY-LEHNEN, (PhD), CNS, GPN, RNP and National PRO, Irish General Practice Nurses Educational Association
Prostate cancer in focus
Prostate cancer is the second most frequent cancer diagnosed in men and the fifth leading cause of death globally. Excluding non-melanoma skin cancer, prostate cancer is the leading cause of cancer in men internationally. In 2018, 1,276,106 new cases of prostate cancer were registered worldwide, representing 7.1 per cent of all cancers in men and 358,989 deaths representing 3.8 per cent of all male cancer deaths.
Approximately 3,890 men are diagnosed with prostate cancer each year in Ireland, indicating that one-in-seven men in Ireland will be diagnosed with prostate cancer during their lifetime (Irish Cancer Society 2021, and National Cancer Registry Ireland (NCRI)).
It accounts for 30 per cent of all newly-diagnosed cancers in Irish males and 11 per cent of all invasive cancers in Ireland (NCRI).
Prostate cancer incidence rates are highly variable worldwide, which is likely to be attributed to different prostate-specific antigen (PSA) testing rates. In Europe, prostate cancer is the most frequently diagnosed cancer among men, accounting for 24 per cent of all new cancers in 2018, with around 450,000 new prostate cancer cases detected in 2018.
Risk factors
The most common risk factor for prostate cancer is increasing age. While one-in-350 men under the age of 50 years will be diagnosed with prostate cancer, the incidence rate increases to one in every 52 men for those aged 50-to-59 years.
Prostate cancer most commonly affects men over the age of 50 and almost two in every three prostate cancers are diagnosed in men over the age of 65 years. In Ireland, the majority of cases are detected in men aged 65-to-84 years, with 37 per cent of cases detected in men under 65 years of age. Genetic factors play a role. Family history is associated with an increased risk and men with a father or brother diagnosed with prostate cancer at age 50 years have an approximately two-fold increased risk of prostate cancer. Risk is higher in males with a relative who developed prostate cancer at a younger age and in males who have more than one relative with the disease. Two breast cancer genes, BRCA1 and BRCA2, have been linked to prostate cancer. Like women, men can have mutations in the BRCA1 and BRCA2 genes. Men carrying mutations in BRCA2 genes have an increased risk of developing prostate cancer, and mutations in either gene can significantly reduce survival. Studies have also revealed an association between hereditary susceptibility to prostate cancer and sequence variations in the RNASEL gene (ribonuclease L), which plays a role in maintaining immunity against viral infections. A common RNASEL variant involves a mutation resulting in decreased activity of the encoded ribonuclease L protein, re-
ducing the immune defence against viruses. Men who inherit this mutation have a significantly increased risk of developing prostate cancer. It is estimated that about 20 per cent of patients with prostate cancer report a family history, which may develop not only because of shared genes, but also for a similar pattern of exposure to certain environmental carcinogens and common lifestyle habits. Afro-Caribbean men have the highest incidence of prostate cancer of any group (231.9 per 100,000) while Asian men have the lowest risk. Obesity and physical inactivity have been associated with higher-grade prostate cancers and studies have shown increased risk associated with various dietary intakes, including high levels of high-saturated fats and red meats, and reduced intake of fish, fruit, and vegetables. Research is ongoing into the links between diet and prostate cancer and there is some evidence that a diet high in calcium is also linked to an increased risk of developing prostate cancer. Although there are no studies that can sufficiently demonstrate the direct correlation between diet and nutrition with risk or prevention of prostate cancer development, many preclinical studies that looked at links between certain eating behaviours and cancer suggest there may be a connection.
Symptoms
When the prostate gland becomes cancerous, it can put pressure on the urethra, causing dysuria, a burning sensation and frequency of micturition. It can also cause hesitancy, a weak and inter-
Staging
Tumour (T) –Size of the tumour
T1 The tumour is within the prostate gland. It is too small to be felt during a rectal exam.
T2 The tumour is still within the prostate gland. It is large enough to be felt during a rectal exam.
T3 The tumour can be felt throughout the prostate and may have broken through the outer layer of the prostate.
T4 The tumour has spread to organs outside the prostate gland.
Node (N) – Are the lymph nodes affected?
N Cancer is present in the lymph nodes.
N0 No cancer in the lymph nodes.
N1 Cancer has spread to one or more of the lymph nodes.
If diagnosed with early prostate cancer, N0 signifies that the
inary diagnosis can be made by rectal examination or transrectal ultrasound (TRUS). A PSA blood test is used to detect prostate tumours in their earliest stages in high-risk individuals. Although originally introduced as a tumour marker for the detection of cancer recurrence, PSA testing became widely adopted as a screening tool for prostate cancer. However, it is not prostate cancer-specific and other prostate conditions, such as BPH or prostatitis, can also affect PSA levels. If prostate cancer is suspected a biopsy is done to confirm the
cancer has not spread outside the prostate.
Metastasis (M) –Has it spread outside the prostate?
M The cancer has spread to lymph nodes and/or other organs, commonly bones.
M0 The cancer has not spread.
If diagnosed with early prostate cancer M0 signifies that the cancer has not spread outside the prostate.
mittent flow, nocturia, haematuria and impotence or sexual dysfunction. Other symptoms include swollen lymph nodes in the groin and pain in the pelvis, hips, back, or ribs. More advanced stages of the disease may present with urinary retention and back pain, as the axis skeleton is the most common site of bony metastatic disease. Prostate cancer should not be confused with benign prostate hyperplasia (BPH), which has similar symptoms and often occurs in older men.
Diagnosis
Prostate cancers usually grow very slowly, and symptoms may not occur for some time. If the prostate is enlarged, a prelim-
diagnosis. When detected early, prostate cancer is very treatable. A large majority of prostate cancers are diagnosed either before they have spread or when they have spread only locally. Survival rates in these cases are very high.
Staging
The TNM staging system refers to the size of the tumour (T), if the cancer has spread to the lymph nodes (N), and if the cancer has spread to other parts of the body – metastasis (M) (Figure 1)
The Gleason Score
The Gleason Score is a grading system used to determine the aggressiveness of pros-
tate cancer and can be used to choose appropriate treatment options. The Gleason Score ranges from 1-5 and describes how much the cancer looks like healthy or abnormal tissue. Most cancers score a grade 3 or higher. Since prostate tumours are often made up of cancerous cells that have different grades, two grades are assigned for each patient. A primary grade is given to describe the cells that make up the largest area of the tumour and a secondary grade is given to describe the cells of the next largest area. If the Gleason Score is written as 3+4=7, it means most of the tumour is grade 3 and the next largest section of the tumour is grade 4. If the cancer is almost entirely made up of cells with the same score, the grade for that area is counted twice to calculate the total Gleason Score. Typical Gleason Scores range from 6-10. The higher the Gleason Score, the more likely that the cancer will grow and spread quickly. Scores of 6 or less describe cancer cells that look similar to normal cells and suggest that the cancer is likely to grow slowly. A score of 7 suggests an intermediate risk for aggressive cancer. Scoring a 7 means that the largest section of the tumour (primary score) scored a 3 or 4. Tumours with a primary score of 3 and a secondary score of 4 have a reasonably good outlook, whereas cancers with a primary Gleason Score of 4 and a secondary score of 3, are more likely to grow and spread. Scores of 8 or higher describe cancers that are likely to spread more rapidly, often referred to as high grade or poorly differentiated.
Treatment
Treatment options for patients with prostate cancer depend on the stage and grade of the cancer and include active surveillance, watchful waiting, hormone therapy, radical prostatectomy, external beam radiotherapy, and brachytherapy. Active surveillance involves a PSA blood test every three months and a digital rectal exam (DRE) every six months for the first
Clinical Urology THE MEDICAL INDEPENDENT | 31 MAY 2021 22
Figure 1: TNM prostate cancer staging score
Prostate cancer has one of the highest survival rates of any type of cancer: 92 per cent of all prostate cancers are found when they are in the early stage and almost 100 per cent of men who have local or regional prostate cancer will survive more than five years after diagnosis
Prostate cancer is one of the most common, but also most successfully treated cancers in men
year, followed by a PSA blood test every six months and a DRE at least once a year. Prostate biopsies and imaging tests may also be done every one-to-three years. Because prostate cancers usually progress slowly, a ‘watchful waiting’ approach rather than immediate treatment may be recommended. This is especially true for patients who are elderly or in otherwise poor health. In patients with intermediate or high-risk localised prostate cancer with a real prospect of long-term disease control and those with locally-advanced disease, radical prostatectomy or radical radiotherapy should be offered.
Hormone therapy is the primary treatment for metastatic prostate cancer, but is also used for patients with locally-advanced, non-metastatic disease. In patients with localised prostate cancer, the choice of treatment depends on whether the disease is low, intermediate, or high risk. Hormone therapy (androgen suppression or androgen deprivation therapy (ADT)) attacks androgens that stimulate the growth of prostate cancer. A form of hormone therapy involves LHRH analogs or LHRH agonists, such as buserelin, goserelin, leuprorelin acetate or triptorelin that chemically block the production of androgens. Side-effects of hormone therapy include reduced libido, sexual dysfunction, osteoporosis, gynaecomastia and hot flushes.
Brachytherapy is a form of radiation therapy used to treat prostate cancer. Prostate brachytherapy procedures vary based on the type. High-dose rate (HDR) brachytherapy is a temporary type of prostate brachytherapy that involves placing radioactive sources in the prostate gland and delivering a high dose of radiation over a few minutes before the sources are removed. Treatment may involve several sessions. Low-dose rate (LDR) brachytherapy is permanent and involves placing radioactive seeds in the prostate gland permanently, where they slowly release radiation over several months. Brachytherapy may be the only treatment used for early-stage prostate cancer that is less likely to spread beyond the prostate. For larger prostate cancers or those that have a greater chance of spreading beyond the prostate, brachytherapy may be used along with other treatments, such as external beam radiation therapy (EBRT) or hormone therapy.
EBRT can be used to try to cure earlier stage cancers, or to help relieve symptoms, such as bone pain if the cancer has spread to a specific area of bone.
Surgery is usually only carried out if the cancer has not spread from the prostate. A radical prostatectomy may be considered if examination of the pelvic lymph nodes reveals that they are not cancerous. Surgical risks can include impotence and urinary incontinence. TURP can be used to relieve symptoms, but does not remove all of the cancer. TURP is often used in men who cannot have a radical prostatectomy because of advanced age or illness or in men who have a non-cancerous enlargement of the prostate. In men who are unable to have traditional surgery, cryosurgery may also be used.
If the cancer has spread from the prostate, radiation therapy may be used. Bi lateral orchidectomy should be offered to all patients with metastatic prostate cancer as an alternative to continuous LHRH agonist treatment. If surgery or hormone therapy fails, chemotherapy may be used. While chemotherapy can slow the growth of the tumour, it is not very effective in treating prostate cancer.
Prognosis and outlook
The outlook for prostate cancer is generally good because, unlike many other types of cancer, it usually progresses very slowly. If treated early, prostate cancer can often be cured. The survival rate is over 90 per cent and many men die with prostate cancer, rather than as a result of having it. Prostate cancer has one of the highest survival rates of any type of cancer: 92 per cent of all prostate cancers are found when they are in the early stages and almost 100 per cent of men who have local or regional prostate cancer will survive more than five years after diagnosis. For most with local or regional prostate cancer, the relative 10-year survival rate is 98 per cent and the relative 15-year survival rate is 96 per cent. Once prostate cancer has spread beyond the prostate, however, survival rates fall and about 7 per cent have more advanced prostate cancer at the time of diagnosis. For men with prostate cancer that has spread to other parts of the body, the five-year survival rate is 30 per cent.
In summary
Prostate cancer presents a number of challenges for primary care clinicians. Many men with prostate cancer are asymptomatic until the tumour has progressed and common symptoms have significant crossover with benign conditions affecting the prostate.
PSA-based testing of prostate cancer is very common but remains controversial. The value of screening remains uncertain because the PSA test does not distinguish between benign and malignant disease and there has been no proof that early treatment leads to increased cure rates. DRE alone is insufficient for screening as its positive predictive value is only 11-to-26 per cent. Current diagnostic tests have limitations in terms of significant false positive and false negative rates, however, research is ongoing into improved methods for diagnosing prostate cancer. Understanding the underlying causes of prostate cancer, including genetics and pathogenesis, has improved sub-
stantially in recent years. Nanotechnology has shown initial success in prostate cancer disease diagnosis, imaging and treatment. A number of new tests and testing strategies are being trialled to improve the diagnosis of clinically significant prostate cancer and blood-based biomarkers for prostate cancer are also being extensively investigated. Because the value of PSA-based testing of prostate cancer remains unclear, more genetic testing–based detection strategies are needed to identify individuals at high risk of prostate cancer and novel drugs need to be evaluated to substantially improve the clinical care of patients with prostate cancer. Continued clinical and translational research in prostate cancer is important and key to the future treatment and management of prostate cancer through leading improvements in prostate cancer imaging and diagnosis.
References on request
doctorCPD.ie
Latest module
Cardiac amyloidosisOverview, Diagnosis and Management
Learning objectives of this module:
• Understand the principal causes (AL amyloidosis and ATTR) and underlying pathophysiology of cardiac amyloidosis
• Identify patients to screen for cardiac amyloidosis and the subsequent diagnostic process
• Develop insight into the management of cardiac amyloidosis and its underlying causes
Urology Clinical THE MEDICAL INDEPENDENT | 31 MAY 2021 23
A B C Free CPD – now accessible on android, iPhone and tablet Successful completion of this module will earn you 2 CPD credits visit www.medilearning.ie/doctorcpd.ie
General practice and cardiology
At the heart of general practice
The General Practice Study Day meeting addressed a range of issues, including managing cardiovascular risk, achieving optimum lipid targets, emerging medical technology, and addiction during Covid-19. The speakers included Dr Susan Connolly, Consultant Cardiologist, Bon Secours Cavan Clinic; Prof Neil Poulter, Professor of Preventative Cardiovascular Medicine, Imperial College London; Prof Derek O‘Keeffe, Consultant Physician at University Hospital Galway and Professor of Medical Device Technology at NUI Galway; and Dr Conor Farren, Consultant Psychiatrist, St Patrick’s University Hospital, Dublin. The meeting, which was facilitated by Servier, was held on a virtual platform and was chaired by Dublin GP Dr Andrew Jordan.
The meeting heard first from Dr Connolly, who delivered a talk titled ‘Achieving Lipid Targets for All Patients’. Dr Connolly presented a number of case studies and data, and told the attendees that when dealing with laboratories, “it is important to request a whole lipid profile,” she said. “Just knowing the total cholesterol itself is of little value. You really need to know the breakdown in terms of triglycerides, HDL and LDL so you can see whether the lipid profile is atherogenic or not.”
The guideline target for LDL cholesterol is now 1.4mmol/L compared to the previous target of 1.8mmol/L and Dr Connolly told the meeting that “the only statins that will achieve a greater than 50 per cent reduction in LDL — which is what you want in your high-risk and very high risk patients — are atorvastatin and rosuvastatin, with atorvastatin at a dose of 40-to-80mg, or rosuvastatin at 20-to-40mg. Any other statins are moderate-intensity and should not be used in patients who are high-risk or very high risk without a good reason.”
Multifactorial risk
Dr Connolly told the meeting: “In primary prevention, do a full lipid profile; the patient doesn’t need to fast — don’t treat the lipid level, but rather treat their risk,” she said. “Calculate the multifactorial risk using whatever tool you like, but don’t get caught out by the certain small percentage of patients who have genetically high cholesterol and are not suitable for assessment with the risk tool.”
She added that while many pharmacists advise patients to take their statins at night-time, atorvastatin, for example, is a long-acting statin that can be taken in the morning when a patient is taking the rest of their drugs, which is an important factor that aids adherence.
“Also, remember that your LDL target varies according to whatever category of risk the patient falls into, so do check which risk they are — are they low, moderate, high or very high risk?” Dr Connolly continued. “The current achievement of LDL targets is poor, not just in Ireland, but in Europe in general and now the LDL tar-
get is even lower at 1.4mmol/L, so we have even more work to do. Adherence using low- or moderate-intensity statins, and perceived side-effects from statins, are big issues,” she continued. “1.4mmol/L is the target for very high-risk patients, and it will not be easy to achieve. I think we need to realise that the majority of our patients who are very high risk will need combination therapy of high-intensity statin therapy and ezetimibe. I am using that combination in the vast majority of my cardiovascular patients.”
Inadequate control
Next to address the meeting was Prof Poulter, who delivered a talk titled ‘Optimising Cardiovascular Risk Management through the use of Single-Pill Combinations’. Prof Poulter told the attendees that the reasons for inadequate control of blood pressure include ineffective drugs; confusion over guidelines; drug costs; side-effects; poor adherence; and physician inertia. “I don’t think we have ineffective drugs, the problem is that we usually only use one drug,” said Prof Poulter. “Monotherapy is usually inadequate to get to current targets… we need to move to using at least two drugs to effectively control our patients’ blood pressure.”
He stressed the need to improve medication adherence to control blood pressure and said: “One of the ways to do that — and this has been in all the guidelines — is by using single-pill combinations. They are more effective in rapid blood pressure control than if you give one drug or two drugs separately,” he continued. “The two drugs separately are not as effective as the same two drugs in a single pill, because often, the patients simply don’t take them.”
“If you use two drugs in a single pill at a lower dose, you get less side-effects and will provide better blood pressure-lowering than one drug at top dose, plus you don’t get the additional side-effects associated with using the top dose of a drug. So we know that with single-pill drugs, patients are more likely to take them and therefore get better cardiovascular protection,” said Prof Poulter. “If there is better cardiovascular protection, inevitably it is more cost-effective, and there is good observational evidence to support all these points.”
Prof Poulter presented overviews of trial data, including studies published in The Lancet on which he was an author. He noted that previous studies showed raised blood pressure continues to be the biggest contributor to the global burden of disease and mortality, leading to 9.4 million deaths each year. “If you take the latest global burden of disease data from 2019, that figure becomes 10.8 million deaths per year,” he said. “That means there are 30,000 deaths every single day due to raised blood pressure. I’m not trying to play down the mortality from Covid, but Covid will never result in 30,000 deaths per day, but the situation with raised blood
pressure will get worse because the world’s population is growing and getting older, so we need to get that in perspective.”
Prof Poulter concluded by stating: “For nearly all our patients, we are going to need two tablets to optimally lower their blood pressure and we will need a statin to add-in to complete cardiovascular protection, so the minimum we need for our blood pressure patients is two antihypertensive agents and a statin and if you can get all three in one tablet, that would be even better, because it will result in better adherence.”
‘Cambrian explosion’
Next to speak was Prof O’Keefe, who presented on the topic ‘Medical Technology in Chronic Disease Management’.
Prof O’Keefe, a physician-engineer, gave an overview of some of the existing and emerging technology that can aid in chronic disease management, including more sophisticated and advanced drones, sleep monitoring devices, and artificial
and perhaps 10 of them actually are, if you have AI helping you to sort through these referrals in the future, you may only get 20 referrals in that week because the AI has screened the other non-melanomas out.”
Prof O’Keefe summarised by saying:
“This med-tech frontier that we find ourselves on, coupled with the ‘Internet of things’ and connected ecosystem of medical devices, will allow us to be better clinicians and for more patient-centered care to evolve that will be less hospital-centric,” he told the meeting. “It will be more about getting the care you need in a timely way in the place where you need it, and from there it can be escalated if necessary.”
Causes of dependence
The final presentation was delivered by Prof Farren, who spoke on the topic ‘Addictions in a Pandemic’. Prof Farren provided a synopsis of data showing the numerous health consequences of alcohol addiction, as well as an overview of self-help options, psychotherapies and appropriate medications. He noted the huge surge in alcohol sales since the beginning of the pandemic and the “potentially life-saving” process of detoxification in treatment.
intelligence (AI), among others. He also outlined some of his work on missions with NASA, which included a mission to the International Space Station, where he conducted medical experiments.
Outlining some of the existing technology, he said: “In dermatology for example, for conditions such as psoriasis and eczema, there are a number of trackers available to monitor patients’ chronic skin conditions as they try different treatments,” said Prof O’Keeffe. “Melanomas can also be photographed and triaged using AI, and these apps are already available in Europe.”
Overall, he described the rapid emergence of new medical technology as a “Cambrian explosion, which goes handin-hand with the new digital medicine frontier of the ‘Internet of things’, as well as connected devices with remote sensing. A lot of people are concerned that perhaps this will make doctors redundant, but it’s actually the opposite,” he continued. “This will make doctors more productive because what it will allow to happen is, it will allow physicians, dietitians and nurses to operate higher up their licence. For example, if you are a dermatologist and you get 100 referrals a week from primary care asking ‘is this a skin cancer?’
Addressing the causes of alcohol dependence, Prof Farren told the meeting: “Genetic alcohol dependence is around 40to-50 per cent [of causes],” he said. “Environment and a permissive culture are also causes… also early exposure to alcohol, and those in their teens and young adults particularly self-medicate with alcohol to deal with social phobia and social interaction. Likewise, a lot of people who suffer with depression take alcohol and even for those that are not social-phobic, they use alcohol as a ‘social lubricant’. Females, particularly, can learn to problematically drink from male heavy-drinking partners.” In addition, personality traits such as novelty-seeking and poor harm avoidance can be associated with a person who is at risk of developing a problem, he added.
Prof Farren gave an overview of some of the support groups available to those who are alcohol-dependent, as well as effective opiate antagonists and strategies to prevent relapse, and psychotherapies that are appropriate to individuals and their circumstances.
“We have developed a smartphone app called UControlDrink, which is a mix between a smartphone and web-based platform,” he told the attendees. “We have found it to be effective, user-friendly, relatively inexpensive and highly personalised,” he said. “It was developed specifically for younger people, but it actually turned out to be effective also for middle-aged and some elderly people.” In addition, there are 12 sessions of Cognitive Behavioural Therapy built into the app, Prof Farren added.
References available on request
This article has been facilitated by Servier. Servier has had no editorial input into the content of this article.

THE MEDICAL INDEPENDENT | 31 MAY 2021 24 Clinical
Article facilitated by
A
recent meeting heard from experts in their fields on managing patients at risk of cardiovascular events in the primary care setting
Monotherapy is usually inadequate to get to current targets… we need to move to using at least two drugs to effectively control our patients’ blood pressure
DUAL CONTROL in one presentation
The 1st Statin & ACEi combination in Ireland2

mg/day and with boceprevir, elbasvir/grazoprevir, the dose of atorvastatin should not exceed 20 mg/day. Elderly and patients with renal failure: frequent monitoring of creatinine and potassium. Clcr < 60 ml/min: not suitable. Hepatic impairment: use with caution. Lipercosyl is contraindicated in patients with active liver disease. Children and adolescents: should not be used.
CONTRAINDICATIONS*: Hypersensitivity to the active substances or to any other ACE inhibitor or statin or to any of the excipients, active liver disease or unexplained persistent elevations of serum transaminases exceeding 3 times the upper limit of normal, during pregnancy, while breast-feeding and in women of child-bearing potential not using appropriate contraceptive measures (see section PREGNANCY* and BREASTFEEDING*), concomitant use with the hepatitis C antivirals glecaprevir/pibrentasvir, history of angioedema associated with previous ACE inhibitor therapy, hereditary or idiopathic angioedema, concomitant use with aliskiren-containing products in patients with diabetes mellitus or renal impairment (GFR < 60 mL/min/1.73 m²) (see section INTERACTIONS* and PHARMACODYNAMIC PROPERTIES* ), concomitant use with sacubitril/valsartan (see WARNING* and INTERACTIONS*), extracorporeal treatments leading to contact of blood with negatively charged surfaces (see INTERACTIONS*), significant bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney (see WARNINGS*). WARNINGS *: Special warnings and precaution for use: Liver effects: liver function tests should be performed periodically and in case of transaminase levels increased, the patient should be monitored until resolution. Stop treatment if jaundice or marked elevations of hepatic enzymes (serum transaminases exceeding 3 times the upper limit of normal) and in patients with active liver disease. Use with caution in patients with hepatic impairment, who consume alcohol and/or have history of liver disease. Skeletal muscle effects: stop treatment if elevation of CK levels > 10 x ULN or muscular symptoms with elevation of CK level > 5 x ULN occur, or if rhabdomyolysis is suspected. Concomitant treatment with other medicinal products: Caution should be exercised when Lipercosyl is used with certain medicinal products that may increase the plasma concentration of atorvastatin and then the risk of rhabdomyolysis such as potent inhibitors of CYP3A4 or transport proteins (e.g ciclosporine, ketoconazole, ritonavir…). Increased risk of myopathy with concomitant use of gemfibrozil and other fibric acid derivates, antivirals for the treatment of hepatitis C, erythromycin, niacin and ezetimibe, the hepatitis C antiviral agents. Co-administration with systemic fusidic acid or within 7 days of stopping the treatment is not recommended. If use is essential, Lipercosyl should be discontinued during fusidic acid treatment. There have been very rare reports of an immune-mediated necrotizing myopathy (IMNM) during or after treatment with some statins. Interstitial lung disease: if suspected, treatment should be discontinued. Diabetes Mellitus: In diabetic patients, glycaemic control should be closely monitored during first month of treatment. Hypotension: monitor blood pressure, renal function and potassium in patients at high risk of symptomatic hypotension (volume depleted or who have severe renin-dependent hypertension) or with symptomatic heart failure (with or without renal insufficiency), or with ischaemic heart or cerebrovascular disease. A transient hypotensive response is not a contraindication to further doses once the blood pressure has increased after volume expansion. Aortic and mitral valve stenosis/hypertrophic cardiomyopathy: use with caution. Kidney transplantation: no experience in case of recent transplantation. Renovascular hypertension: Increased risk of hypotension and renal insufficiency in patient with bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney. Diuretics may be a contributory factor. Loss of renal function may occur (minor changes in serum creatinine) even in patients with unilateral renal artery stenosis, Renal impairment: monitor potassium and creatinine; individual dose titration with the monocomponents recommended if Clcr < 60 ml/min. In patients with renal artery stenosis, blood urea and creatinine may increase; in patients with renovascular hypertension, increased risk of severe hypotension and renal insufficiency. Haemodialysis patients: anaphylactoid reactions have been observed when high flux membrane were used; different type of membrane should be considered. Hypersensitivity/Angioedema: stop treatment and monitor until complete resolution of symptoms. Angioedema associated with laryngeal oedema may be fatal. Combination with sacubitril/valsartan (contraindicated due to the increased risk of angioedema). Sacubitril/valsartan must not be initiated until 36 hours after taking the last dose of perindopril therapy. Perindopril therapy must not be started until 36 hours after the last dose of sacubitril/valsartan. Concomitant use of other NEP inhibitors (e.g. racecadotril) and ACE inhibitors may also increase the risk of angioedema. Concomitant use of mTOR inhibitors: Increased risk for angioedema. Anaphylactoid reactions during low-density lipoproteins (LDL) apheresis: temporarily withhold treatment prior to each apheresis. Anaphylactoid reactions during desensitisation: temporarily withhold treatment prior to desensitisation. These reactions reappeared upon inadvertent rechallenge. Neutropenia/agranulocytosis/thrombocytopenia/ anaemia: extreme caution in patients with collagen vascular disease, immunosuppressant therapy, treated with allopurinol or procainamide, periodic monitor of white blood cell counts advised. Race: perindopril may be less effective and cause a higher rate of angioedema in black patients than in non-black. Cough: non-productive, resolves after discontinuation. Surgery/Anaesthesia: stop treatment one day prior to surgery. Hyperkalaemia: frequent monitoring of blood potassium if renal insufficiency, worsening of renal function, age (>70 years), diabetes mellitus, dehydration, acute cardiac decompensation, metabolic acidosis, and concomitant use of potassium-sparing diuretics and potassium salts or supplements or other drugs associated with increases in serum potassium (e.g. heparin, co-trimoxazole). Combination with lithium: not recommended. Dual blockade of the renin-angiotensin-aldosterone system (RAAS): concomitant use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren increases the risk of hypotension, hyperkalaemia and decreased renal function (including acute renal failure). Dual blockade of RAAS is therefore not recommended. ACE-inhibitors and angiotensin II receptor blockers should not be used concomitantly in patients with diabetic nephropathy. Primary aldosteronism: Use not recommended in patients with primary hyperaldosteronism (not responding to drugs acting through inhibition of renin-angiotensin system). Fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency: should not be taken. Sodium: Lipercosyl essentially ‘sodium-free’.(less than 1 mmol sodium (23 mg) per capsule). INTERACTION(S)*: Contraindicated: Aliskiren (in diabetic and impaired renal patients), Extracorporeal treatments, Sacubitril/valsartan, Glecaprevir/pibrentasvir. Not recommended potent CYP3A4 inhibitors, Inhibitors of Breast Cancer Resistant Protein (BCRP), aliskiren (in patients other than diabetic or impaired renal patients), Co-trimoxazole (trimethoprim/ sulfamethoxazole), concomitant therapy with ACE inhibitor and angiotensin-receptor blocker, estramustine, lithium, potassium-sparing diuretics (e.g. triamterene, amiloride, eplerenone, spironolactone), potassium salts, grapefruit or grapefruit juice. Special care: moderate CYP3A4 inhibitors, CYP3A4 inducers, digoxin, ezetimibe, fusidic acid, gemfibrozil / fibric acid derivatives, transport inhibitors, warfarin, antidiabetic agents (insulins, oral hypoglycaemic agents), baclofen, non-potassium-sparing diuretics, racecadotril, mTOR inhibitors (e.g. sirolimus, everolimus, temsirolimus), non-steroidal anti-inflammatory medicinal products (NSAIDs) (including aspirin ≥ 3 g/day). To be taken into consideration: colchicine, colestipol, oral contraceptives, gliptins (linagliptin, saxagliptin, sitagliptin, vildagliptin), sympathomimetics, tricyclicantidepressants/antipsychotics/anesthetics, gold, antihypertensive agents and vasodilators. PREGNANCY AND BREASTFEEDING*: Lipercosyl is contraindicated during pregnancy, while breast-feeding and in women of child-bearing potential not using appropriate contraceptive measures. FERTILITY*. DRIVE & USE MACHINES*: Low blood pressure may occur in some patients. UNDESIRABLE EFFECTS*: Common nasopharyngitis, hypersensitivity, hyperglycaemia, dizziness, headache, dysgeusia, paraesthesia, visual impairment, tinnitus, vertigo, hypotension (and effects related to hypotension), pharyngolaryngeal pain, epistaxis, cough, dyspnoea, nausea, vomiting, abdominal pain upper and lower, dyspepsia, diarrhoea, constipation, flatulence, rash, pruritus, joint swelling, pain in extremity, arthralgia, muscle spasms, myalgia, back pain, asthenia, liver function test abnormal, blood creatine kinase increased. Uncommon: eosinophilia, hypoglycaemia, hyponatraemia, hyperkalaemia reversible on discontinuation, anorexia, insomnia, mood altered, sleep disorder, nightmares, somnolence, syncope, hypoaesthesia, amnesia, vision blurred, tachycardia, palpitations vasculitis, bronchospasm, dry mouth, pancreatitis, eructation, hepatitis either cytolytic or cholestatic, urticaria, hyperhidrosis, alopecia, angioedema, pemphigoid, photosensitivity reactions, neck pain, muscle fatigue, renal failure, erectile dysfunction, fatigue, chest pain, malaise, oedema peripheral, pyrexia, blood urea increased, blood creatinine increased, weight increase, white blood cells urine positive, fall. Rare: Thrombocytopenia, peripheral neuropathy, cholestasis, psoriasis aggravation, stevens-johnson syndrome, toxic epidermal necrolysis, erythema multiforme, myopathy, myositis, rhabdomyolysis, muscle rupture, tendinopathy sometimes complicated by rupture, hepatic enzymes increased, blood bilirubin increased. Very rare: Rhinitis, leucopenia/neutropenia, agranulocytosis/pancytopenia, haemolytic anaemia in patients with a congenital deficiency of G-6PDH, anaphylaxis, confusional state, stroke possible secondary to excessive hypotension in high-risk patients, hearing loss, myocardial infarction secondary to excessive hypotension in high-risk patients, angina pectoris, arrhythmia, eosinophilic pneumonia, hepatic failure, lupus-like syndrome, acute kidney injury, gynaecomastia, haemoglobin decreased and haematocrit decreased. Not known immune-mediated necrotizing myopathy, Raynaud’s phenomenon. Syndrome of inappropriate antidiuretic hormone secretion (SIADH) can be considered as a very rare complication associated with ACE inhibitor therapy. OVERDOSE*. PROPERTIES*: Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase. Perindopril is an inhibitor
Lipercosyl® (Atorvastatin/Perindopril): Abbreviated Prescribing Information: Please refer to the Summary of Product Characteristics before prescribing. COMPOSITION*: Lipercosyl 10 mg/5 mg, 20mg/5mg, 40 mg/5 mg, 10 mg/10 mg, 20 mg/10 mg, 40 mg/10 mg, hard capsules contains 10 mg atorvastatin (ator)/5 mg perindopril arginine (per), 20 mg ator/5 mg per, 40 mg ator/5 mg per, 10 mg ator/10 mg per, 20 mg ator/10 mg per, 40 mg ator/ 10mg per. Contains sucrose as excipient. INDICATIONS*: Substitution therapy as part of cardiovascular risk management in adult patients adequately controlled with atorvastatin and perindopril given concurrently at the same dose level but as separate products. DOSAGE AND ADMINISTRATION*: One capsule once daily in the morning before a meal. Lipercosyl is not suitable for initial therapy. If a change of posology is required, titration should be done with individual components. Standard cholesterol-lowering diet should be continued during treatment. Co-administration with tipranavir, ritonavir, telaprevir or ciclosporine the dose of atorvastatin should not exceed 10
of the enzyme that converts angiotensin I into angiotensin II (Angiotensin Converting Enzyme ACE). PRESENTATION*: Box of 30 hard capsules for Lipercosyl 10mg/5mg, 20mg/5mg, 40mg/5mg, 10mg/10mg, 20mg/10mg, 40mg/10mg. LES LABORATOIRES SERVIER 50 rue Carnot, 92284 Suresnes cedex France. www.servier.com. Marketing Authorisation: PA0568/032/001-006. Legal classification for supply: POM. Local Representative in Ireland: Servier Laboratories (Ireland) Ltd. Second Floor, 19 Lr. George’s Street, Dun Laoghaire, Co. Dublin A96 ER84, Ireland, Tel (01) 6638110, www.servier.ie * For complete information, please refer to the Summary of Product Characteristics available on www.medicines.ie Date of last revision of text: September 2020 (date of last approved SmPC: December 2019). 2021 LPC Press ad. Date of Preparation September 2020. 1. Lipercosyl SmPC, Dec 2019. 2. IMS, C11A Monthly Unit Sales, July 2020. Controlling High Cholesterol and Hypertension in tandem1 NEW
PAUL NOLAN, Chief II Cardiac Physiologist, Associate Academic Officer, Cardiac Investigations, Saolta University Healthcare Group, University Hospital Galway
Sláintecare Galway University Hospital Community Cardiac Diagnostics Service

In recent years in Irish healthcare, there has been focus on shifting more care into the community, closer to patients’ homes. This is reinforced in the Sláintecare Report and the intended approach is embodied by the ‘right care, right place, right time’ mantra.
There has long been recognition of the difficulty that GPs have in accessing diagnostics. A report from the ICGP demonstrated that:
<20 per cent of GPs have direct access to echocardiography;
<25 per cent of GPs have direct access to heart monitoring (eg, Holter/R-test);
86.7 per cent of GPs agree or strongly agree that improved access to diagnostics would reduce their referrals to emergency departments (EDs).
Indeed, one of the effects of this lack of access to diagnostics is that GPs often have little option but to refer to outpatient clinics, with long wait times, or acute services such as the ED or Acute Medical Units. Direct access for GPs to diagnostics addresses these needs and meets the Sláintecare vision of shifting care appropriately, away from a hospital-centric model.
Mobile diagnostics are not new; indeed in October 1914 the first ‘petite Curie’, brought x-rays to the frontlines of World War I (Figure 1). Community cardiac diagnostics have been running in the UK for a significant period, with a positive impact for patients. It is reported that they prefer attending less stressful and more accessible clinics, and patient-reported experience measures are compelling. A similar community-based cardiac diagnostics service in Sligo demonstrated a saving of 250 bed days over a six-week period, by facilitating early discharge from hospital with follow-up diagnostics being delivered in the community.

Sláintecare Integration Fund
In 2019, all healthcare staff and organisations were invited to submit ideas to Sláintecare to access some of a €20 million Integration Fund. There was a particular emphasis on initiatives that would support the shift to community-based care and help to reduce and prevent hospital visits. The cardiology department in Galway University Hospital was successful in its application and received Sláintecare funding to commence its Community Cardiac Diagnostics Programme. This funding allowed us to purchase a portable echocardiography machine, of an equivalent standard to the hospital-based machine and 10 heart (R-test) monitors. It also covered the salary of a senior cardiac physiologist, who performs and reports the echocardiograms, and
an administrative post.
The service currently operates from five clinics in Tuam, Claremorris, Gort, and two in Galway city. These sites are reachable by staff within 45 minutes, thus maximising the number of diagnostic slots available. The service is currently providing direct access for GPs to 40 diagnostic slots per week.
The service is provided by the team of cardiac physiologists, under the clinical support of the consultant cardiologists from Galway University Hospital, rotating out to the centres. The cardiac physiologists providing the service have internationally recognised accreditation in echocardiography. This combined with it being under the clinical governance of the hospital gives associated quality assurance. The clinical support and governance of the consultant cardiologists is vital, in terms of their expertise for more difficult cases and continuity of care in referral pathways.
All images and reports of investigations performed are archived to the hospital’s cardiovascular IT systems and uploaded to the hospital’s electronic chart. This ensures continuity of care in the case of referral to the hospital at a future date. It also ensures that tests are not repeated unnecessarily.
One of the initiatives the cardiology team worked on was to create a GP summary template for various findings. The aim of these was to translate what can be quite a technical echocardiogram report into a summary with clear guideline-driven recommendations; for example, in cases of mild aortic stenosis, with no calcification, we recommend to repeat the Echo in two-to-three years. The aim of this is to make it easier for GPs to manage the patient and their results. In the case of significant findings requiring more urgent referral, our cardiology team will arrange this with the GP advised of this.
Indications
Typical indications for echocardiography from primary care would include:
Murmur
l Symptomatic murmur.
l Asymptomatic murmur with other signs suggestive of structural heart disease.
Suspected heart failure
l Clinical signs of heart failure.
l Unexplained shortness of breath if ECG/CXR abnormal.
l With higher priority given to referrals with elevated BNP.
Syncope
l With clinical suspicion of cardiac disease.
l Exertional syncope.
l New onset, rate controlled, atrial fibrillation.
l Palpitations.
The R-test monitor records a full heart rate trend and automatically records arrhythmias, including everything from ventricular ectopic to atrial fibrillation and ventricular tachycardia. Indications for R-test (similar to Holter) include:
Syncope and presyncope, where it is unlikely to be vasovagal.
Palpitations
l Symptomatic and unlikely to be ectopic beats. Palpitations can be a troublesome diagnostic challenge and can cover everything from a patient being very aware of their heartbeat, to ectopic beats, to a significant arrhythmia. While a normal resting 12-lead ECG gives some degree of reassurance, the diagnosis is made by getting a symptom/rhythm correlation. It has been shown that 24to-48 hour ambulatory monitoring has a relatively low diagnostic yield unless the patient has daily palpitations and whilst event monitors have a higher diagnostic yield, they are typically only fitted for a couple of weeks. New technology, such as hand-held ECGs, for example, the Alivecor device, have a high yield for a relatively low, once-off cost.
In the setting of palpitations, a normal echocardiogram can be reassuring. Where the nature of the palpitations has not yet been characterised, a normal Echo and a normal ECG would suggest no further management is required. If the palpitations were very frequent or distressing, referral could be considered.
Some signs, which would point to an increased likelihood of a significant arrhythmia, include:
Symptoms at work;
Exertional symptoms;
Symptoms disturbing sleep;
Known cardiovascular disease;
Clinical Cardiology THE MEDICAL INDEPENDENT | 31 MAY 2021 26
Figure 2: GUH Community Cardiac Diagnostics Programme data to-date
Figure 1: A ‘petite Curie’, mobile x-ray vehicle from WWI
Older age;
On cardiovascular medication.
Service to-date
The Sláintecare GUH Community Cardiac Diagnostic Clinic has seen over 290 patient in the first two months of 2021, performing 216 echocardiograms and 75 R-test monitors (Figure 2). A total 87 per cent of tests were completed within six weeks of referral and over half were seen in two weeks or less.
Over a quarter (28 per cent) of tests have a significant abnormality that would require follow-up, but in 59
CASE STUDY
An 83-year-old woman was recently seen by her GP, and although she had no concerning cardiovascular symptoms, the GP noted an ejection systolic murmur. This lady had previously had an Echo, which showed aortic sclerosis and recommended repeat echo in five years. Given the presence of the murmur and the previous Echo, the patient was referred for echocardiography.
Her referral was received via Healthlink on January 28 and this was performed in our Tuam clinic on February 28 and revealed severe aortic stenosis. Echocardiography can classify the severity of valve narrowing by measuring the pressure gradient across the valve and through a combination of measurements estimate the aortic valve area (AVA). Severe aortic stenosis is classified as:
Peak velocity across the valve of >4.0m/s –the narrower the valve, the faster the blood flow;
Mean pressure gradient of >40mmHg;
Estimated aortic valve area of <1.0cm2 –normal area is 2-4cm2
The patient was referred to our structural heart clinic. There is ample evidence that in patients with symptomatic severe aortic stenosis that valve replacement improves survival, symptoms, and overall cardiac function.
There are two intervention options for patients with severe aortic stenosis – surgical valve replacement (SAVR) or transcatheter aortic valve replacement (TAVI), where the valve is delivered via a catheter from the femoral artery in a non-surgical approach. Current guidelines support the use of TAVI in patients who are either too high risk or high risk for SAVR. In patients with lower surgical risk, decisions are made based on issues, such as valve durability, procedural difficulty, co-morbidities and patient preference. The overall recommendation is made by a multidisciplinary heart team, including cardiologists and cardiac surgeons.
These guidelines, based on the evidence that asymptomatic patients with severe AS and preserved ejection fraction have a similar survival to age matched cohorts, also recommend following up these patients at six-monthly intervals, with prompt intervention at the onset of symptoms, such as shortness of breath, reduced exercise tolerance, pre-syncope or syncope, or a reduction in ejection fraction.
When the patient was seen in the clinic, her functional status was unclear. As aortic stenosis is a degenerative valvular disease, a patient’s functional capacity can decline gradually, unnoticed by the patient. She had an exercise test performed to quantify her exercise capacity and this was reassuring and will be followed up in the structural heart clinic in six months’ time with a repeat echocardiogram.
Via the new Sláintecare Galway University Hospital Cardiac Diagnostics Service, the patient was diagnosed, seen in a specialist clinic and managed within 10 weeks from referral.
per cent of these cases the follow-up could be left with the GP, furnished with a recommendation in the diagnostic report.
Significant findings that required onward referral by the service directly included:

Reduced left ventricular function;
Significant valve disease;
Severe pulmonary hypertension;
Newly discovered paroxysmal atrial fibrillation.
Future direction
The service has been widely adopted by GPs with high levels of satisfaction from patients. It is leading to more timely diagnosis as outlined in the associated case study.
One 85-year-old patient who attended our Gort clinic sums up the improved patient experience. She is reliant on taxis to take her to hospital appointments, which is
an over 90km round trip and a taxi fare of €110. The ability to attend our community clinic transformed this to a five-minute trip.
With such benefits in terms of wait times, earlier diagnosis, and improved patient experience – we are hopeful that funding for the service will be continued and perhaps expanded as community-based integrated care hubs are rolled out nationally.
This service is dependent on the support of the primary care centres, Community Health Organisations, Galway University Hospital management and the Saolta Project Management Office. The key element is the hard work and support of the team of cardiac physiologists, cardiac consultants and cardiology administration staff in Galway University Hospital.
References on request
IE Prescribing Information: Targaxan 550mg (rifaximin-α)


REFER TO FULL SUMMARY OF PRODUCT CHARACTERISTICS (SmPC) BEFORE PRESCRIBING
Presentation: Film-coated tablet containing rifaximin 550 mg. Uses: Targaxan is indicated for the reduction in recurrence of episodes of overt hepatic encephalopathy in patients ≥ 18 years of age. Dosage and administration: Recommended dose: 550mg twice daily as long term treatment for the reduction in recurrence of overt episodes of overt hepatic encephalopathy. In the pivotal study, 91% of patients were using concomitant lactulose. TARGAXAN can be administered with a glass of water, with or without food. No dosage changes are necessary in the elderly or those with hepatic insufficiency. Use with caution in patients with renal impairment The safety and efficacy in paediatric patients (aged less than 18 years) have not been established. Contraindications: Contraindicated in hypersensitivity to rifaximin, rifamycin-derivatives or to any of the excipients and in cases of intestinal obstruction. Warnings and precautions for use: The potential association of rifaximin treatment with Clostridium difficile associated diarrhoea and pseudomembranous colitis cannot be ruled out. The administration of rifaximin with other rifamycins is not recommended. Rifaximin may cause a reddish discolouration of the urine. Use with caution in patients with severe (Child-Pugh C) hepatic impairment and in patients with MELD (Model for End-Stage Liver Disease) score > 25. In hepatic impaired patients, rifaximin may decrease the exposure of concomitantly administered CYP3A4 substrates (e.g. warfarin, antiepileptics, antiarrhythmics, oral contraceptives). Both decreases and increases in international normalized ratio (in some cases
with bleeding events) have been reported in patients maintained on warfarin and prescribed rifaximin. If co-administration is necessary, the international normalized ratio should be carefully monitored with the addition or withdrawal of treatment with rifaximin. Adjustments in the dose of oral anticoagulants may be necessary to maintain the desired level of anticoagulation. Ciclosporin may increase the rifaximin Cmax. This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodiumfree’. Pregnancy and lactation: Rifaximin is not recommended during pregnancy. The benefits of rifaximin treatment should be assessed against the need to continue breastfeeding. Side effects: Common effects reported in clinical trials are dizziness, headache, depression, dyspnoea, upper abdominal pain, abdominal distension, diarrhoea, nausea, vomiting, ascites, rashes, pruritus, muscle spasms, arthralgia and peripheral oedema. Other effects that have been reported include: Clostridial infections, urinary tract infections, candidiasis, pneumonia cellulitis, upper respiratory tract infection and rhinitis. Blood disorders (e.g. anaemia, thrombocytopenia). Anaphylactic reactions, angioedemas, hypersensitivity. Anorexia, hyperkalaemia and dehydration. Confusion, sleep disorders, balance disorders, convulsions, hypoesthesia, memory impairment and attention disorders. Hypotension, hypertension and fainting. Hot flushes. Breathing difficulty, pleural effusion, COPD. Gastrointestinal disorders and skin reactions. Liver function test abnormalities. Dysuria, pollakiuria and proteinuria. Oedema. Pyrexia. INR abnormalities. Prescribers should consult the SmPC in relation to all adverse reactions.
IRELAND
Legal category: Prescription only Cost: €262.41 for 56 tablets Marketing Authorisation holder:
Norgine B.V. Antonio Vivaldistraat 150, 1083 HP, Amsterdam, Netherlands Marketing Authorisation number: PA 1336/009/001 For further information contact: Norgine Pharmaceuticals Limited Norgine House Widewater Place, Moorhall Road, Harefield, Uxbridge, UB9 6NS, UK Telephone: +44 (0)1895 826 606 E-mail: Medinfo@norgine.com Ref: IE-HEP-XIF-2100010
Date of preparation: April 2021
Ireland Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance.
Website: www.hpra.ie. Adverse events should also be reported to Medical Information at Norgine Pharmaceuticals Ltd on: Tel. +44 (0)1895 826 606 Email. Medinfo@norgine.com
References:
1. National Institute for Health and Care Excellence. Rifaximin for preventing episodes of overt hepatic encephalopathy: appraisal guidance TA337 for rifaximin. Available from: http://www.nice.org.uk/guidance/ta337


2. TARGAXAN® 550 Summary of Product Characteristics. Available for Ireland from: www.medicines.ie


Product under licence from Alfasigma S.p.A. TARGAXAN is a registered trademark of the Alfasigma group of companies, licensed to the Norgine group of companies. NORGINE and the sail logo are registered trademarks of the Norgine group of companies.
IE-HEP-XIF-2100002

Date of preparation: May 2021

Cardiology Clinical THE MEDICAL INDEPENDENT | 31 MAY 2021 27
Long-term prophylaxis in hepatic encephalopathy (HE)2 The only licensed treatment for the reduction in recurrence of episodes of overt hepatic encephalopathy ≥ 18 years1
At home they are still at risk; …TARGAXAN® rifaximin-α reduces the risk of recurrence of overt hepatic encephalopathy.2
Irish Society of Gastroenterology, Summer Meeting, virtual meeting, 18 June 2021
The latest developments in gastroenterology
Niamh Cahill speaks to President of the Irish Society of Gastroenterology

upcoming virtual meeting, developments in the specialty, and the effect


T
The day-long meeting begins at 9.30am and will see Irish and international specialists deliver talks on subjects including haemochromatosis, cirrhosis and Crohn’s disease, among many other subjects of interest.
Dr Tony Tham about the Society’s
of Covid-19 on services
he Irish Society of Gastroenterology (ISG) Summer Meeting takes place virtually on 18 June with nine presentations by expert speakers on topics pertinent to the specialty of gastroenterology.Speaking to the Medical Independent in advance of the meeting, ISG President Dr Tony Tham said that following the emergence of Covid-19 all face-to-face meetings planned by the Society were switched to a virtual format.
Over the past 12 months the Society has hosted three webinars, which have highlighted some of the main areas within the specialty, such as endoscopy, inflammatory bowel disease, and liver disease, said Dr Tham.
During this time the Society has continued its work in
Colitis1
advancing service improvements in gastroenterology and hepatology in Ireland.
As Chairperson of the HSE National Clinical Programme for Gastroenterology and Hepatology Clinical Advisory Group, Dr Tham welcomed recent approval for six new consultant gastroenterologist posts in Ireland.
The clinical advisory group, set up by the RCPI, oversees the work of the HSE Clinical Programme for Gastroenterology and Hepatology, which is led by Prof Colm O’Morain.
Increasing the number of consultant gastroenterologists in Ireland was one of the group’s strategic objectives, explained Dr Tham.
A 2020 HSE report, 'Demand for medical consultants and specialists to 2028 and the training pipeline to meet demand: A high-level stakeholder informed analysis', showed that in 2018 there were 85 (69 public and 16 private only) consultants working in gastroenterology. Of this number, a fifth were over 55 years of age.
In 2018 the number of higher specialist training trainees working in the specialty was 45. Ireland has 1.8 gastroenterologists per 100,000 of the population compared to 2.5 in England, 2.1 in Scotland, and 2.8 in Australia.
... a central hospital would have a network arrangement with a smaller hospital so that patient treatment could be transferred from the smaller hospital to the larger hospital if required
Specialist demand estimates show that the specialty requires a total head count of 152 gastroenterologists by 2028.
MMX technology enables targeted release of budesonide throughout the entire colon.1,5–8
IN ULCERATIVE COLITIS:
• CORTIMENT, with low systemic bioavailability, has a comparable side effect profile to placebo.1,5–8


• Symptom resolution may be achieved with CORTIMENT in a median of 30 days.9
• Real World Evidence and an RCT demonstrate that CORTIMENT is an effective add-on therapy in mild to moderate active UC patients.9,10
treatment is not sufficient and induction of remission in patients with active Microscopic Colitis. Dosage: Adults: one 9 mg tablet in the morning for up to 8 weeks, swallowed whole with a glass of water. When treatment is discontinued, it may be useful to gradually reduce the dose. Children:No data are available, therefore the use in paediatric population is not recommended. Contraindications: Hypersensitivity to the active substance, soya oil, peanut oil or to any of the excipients of the product. Special Warnings and Precautions: Caution is recommended in hepatic/renal impairment, infections, hypertension, diabetes mellitus (or family history), osteoporosis, peptic ulcer, glaucoma (or family history) or cataracts or with any other condition where the use of glucocorticoids may have unwanted effects. Visual disturbances – consider referral to ophthalmologist if patient presents with symptoms such as blurred vision or other visual disturbances (refer to SPC). Reduced liver function may affect the elimination of glucocorticoids, be aware of possible systemic side effects. Consider gradual withdrawal if discontinuing treatment. Treatment with Cortiment tablets results in lower systemic steroid levels than conventional oral glucocorticoid therapy. Transfer from other steroid therapy may result in symptoms relating to the change in systemic steroid levels. Co-administration of Cortiment tablets is likely to reduce the immune response to vaccines. Concomitant administration of ketoconazole or other potent CYP3A4 inhibitors, including grapefruit juice, should be avoided (see SPC for further information). Cortiment contains lecithin (soya oil). Avoid if hypersensitive to peanut or soya. Contains lactose. Warningsgenerally identifiedforcorticosteroids:adrenocortical suppression (transfer from systemic treatment); increased susceptibility to infections; surgery/other stresses – supplementary systemic corticosteroid treatment recommended; avoid exposure to chicken pox and measles, especially if not previously exposed; systemic
effects may occur (see SPC); current or history of severe affective disorders (including first degree relatives).
See SPC for full information on warnings and precautions. Interactions: No interaction studies have been performed. Inhibitors of CYP3A4 enzyme are expected to increase systemic exposure to budesonide several times and the risk of systemic side effects. Combination should be avoided unless the benefit outweighs the increased risk. CYP3A4 inducers may reduce budesonide exposure. Please refer to the SPC for further information. Pregnancy, lactation and fertility: Cortiment should only be used during pregnancy if the potential benefit justifies the potential risk to the foetus. Lactation: data supports continued use, see SPC.
Fertility: see SPC. Effects on ability to drive and use machines: No studies; occasional dizziness or tiredness may occur. Side effects: Fromclinicaltrials:Common:insomnia, headache, nausea, abdominal pain upper, abdominal distension, abdominal pain, dry mouth, dyspepsia, acne, myalgia, fatigue, blood cortisol decreased.
Uncommon:influenza, leukocytosis, mood altered, dizziness, flatulence, back pain, muscle spasms, oedema peripheral. Therapeuticclasseffects:Common:cushingoid features, hypokalemia; behavioural changes such as nervousness, insomnia and mood swings; depression, palpitations, dyspepsia, skin reactions (urticaria, exanthema), muscle cramps, menstrual disorders. Uncommon:psychomotor hyperactivity, anxiety, tremor.
Dr Tham, who is Consultant Physician and Gastroenterologist based at Ulster Hospital, Belfast, said another objective of the group is to establish a hepatology hub and spoke network throughout the country to improve patient care.
“For example, a central hospital would have a network arrangement with a smaller hospital so that patient treatment could be transferred from the smaller hospital to the larger hospital if required. The expertise from the central hospitals could also go out to the smaller hospitals and deliver local care,” he explained.
The network would make hepatology care more accessible and create integrated care pathways for patients suffering from gastro-intestinal diseases to access care for complaints such as liver failure and inflammatory bowel disease, as well as nutrition, paediatrics, and some endoscopy services.
The network is a “work in progress”. An agreed plan has yet to be devised before a business case is presented to the HSE for approval.
“We are making progress. A few years ago we started off with nothing and now we have a programme lead in Colm O’Morain who is bringing everyone together.”
(B).
Marketing Authorisation Number: PA 1009/26/1 Marketing Authorisation Holder: Ferring Ireland Ltd., United Drug House, Magna Drive, Magna



New developments
Some very exciting developments have occurred within the specialty in recent times, said Dr Tham.
The faecal immunochemical test (FIT) test, currently being rolled out in primary and secondary care services in Northern Ireland, is one such innovation. It is already in
Conference Preview THE MEDICAL INDEPENDENT | 31 MAY 2021 28
HCP; healthcare professional, RCT; randomised controlled trial
Prescribe CORTIMENT 9 mg once-daily for up to 8 weeks to navigate your flaring UC and Active MC patients back into remission.1,7–9 NEW LICENCE for the treatment of Active Microscopic
Abbreviated Prescribing Information Please consult the full Summary of Product Characteristics before prescribing. Name of Product: Cortiment 9 mg prolonged release tablets. Composition: One prolonged release tablet contains 9 mg budesonide. Indication: Induction of remission in patients with mild to moderate active Ulcerative Colitis where 5-ASA
CORTIMENT MMX®: The only oral budesonide licensed for the treatment of both mild to moderate Ulcerative Colitis flares when 5- ASA is insufficient, and Active Microscopic Colitis1–4 9 mg tablet once-daily for up to 8 weeks
Business Park, Citywest Road, Dublin 24. Further
available on request from: Ferring Ireland Ltd., United Drug House, Magna Drive, Magna Business Park, Citywest Road, Dublin 24. Tel: (01) 4637355; Email: enquiries.ireland@ferring.com Cortiment, FERRING and the FERRING Logo are trademarks of Ferring B.V. UK-COR-2000033 Date of Preparation: December 2020.
Rare:aggression, cataract including subcapsular cataract, glaucoma, vision blurred, ecchymosis. Very rare:anaphylactic reaction, growth retardation in children. Please consult the SPC for full information on side effects. Nature and Contents of Container: Blister packs with aluminium push through foil, contained in a cardboard carton. Pack of 30. Legal Category: Product subject to prescription which may be renewed
information is
Date of preparation: March 2021. Job Code: UK-COR-2100007. References: 1. Cortiment 9 mg, Prolonged Release Tablets. SmPC. 2. Tillotts Pharma Budesonide 3 mg Capsules. SmPC. 3. Dr Falk Budesonide 3 mg Gastro-resistant Capsules. SmPC. 4. Dr Falk Budesonide 9 mg Gastro-resistant Granules. SmPC. 5. Brunner M, et al. Br J Clin Pharmacol. 2006;61(1):31–8. 6. Fiorino G, et al. Curr Med Chem. 2010;17(17):1851–7. 7. Travis SPL, et al. Gut. 2014;63:433–41. doi:10.1136/ gutjnl-2012-304258. 8. Sandborn WJ, et al. Gastroenterology. 2012;143:1218–1226. 9. Danese S, et al. J Crohns Colitis. 2019;13 (supplement 1):296–297. 10. Rubin D, et al. J Crohns Colitis. 2017;11(7):785–791. CORP-2021-008_IRELAND_Cortiment_240x165_Medical_Independent.indd 1 06/05/2021 09:24
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie.
use by the HSE as part of the BowelScreen, the national bowel screening programme.
Consultant Gastroenterologist at the University of Warwick in the UK, Prof Ramesh Arasaradnam will deliver a talk entitled “Using FIT in symptomatic disease” at the summer meeting.
FIT testing can be used to triage patients with lower gastrointestinal symptoms. It is a stool test used to detect a patients’ risk of serious underlying disease, said Dr Tham.
Another promising development is the cytosponge, which is used to detect early or pre-cancerous lesions in Barret’s oesophageal cancer, said Dr Tham. This is a new test in development that has yet to be rolled out clinically, he added.
The topic of early gastric cancers and pre-cancer detection and management will be discussed in a talk by Prof Mário Dinis-Ribeiro, a Consultant Gastroenterologist from the Institute of Porto, Portugal, at the summer meeting.
A colon capsule is another potentially significant and innovative test within the specialty that is currently under development, said Dr Tham.

“The colon capsule will go into the colon and take pictures of the colon, so it may be an alternative to colonoscopy.”
Agenda
According to Dr Tham: “It’s important to keep educated and our talks are aimed at the jobbing gastroenterologist and common diseases and how best to manage them for patients.”
The meeting opens with Prof Dinis-Ribeiro’s talk, outlined above. He will be followed by Mr Paul Carroll, Oesophagogastric and General Surgeon, Galway University Hospital, who will give a talk on the future of gastric surgery.
Next up is a presentation on the gastrointestinal consequences of pelvic radiation disease by Prof Jervoise Andreyev, Consultant Gastroenterologist and Honorary Professor at the University of Nottingham, UK.
He will be followed by Dr David Kevans, Consultant Gastroenterologist at St James’s Hospital, Dublin, who will look at preventing post-operative recurrence of Crohn’s disease.
A talk on what’s new in haemochromatosis by Prof Susanne Norris, Consultant Hepatologist, St James’s Hospital, Dublin, begins after the lunch break.
Managing ascites in cirrhosis is the next topic under review by Prof Guru Aithal, Professor of Hepatology and Head of Division for Digestive Diseases at the University of Nottingham, UK.
Dr Eamonn Quigley and past president of WGO, Chief of Gastroenterology and Hepatology at Houston Methodist Hospital, Texas, US, will then provide an overview of oesophageal dysmotility.
Prof Arasaradnam’s talk on FIT testing, mentioned above, precedes the final presentation of the day, which will outline the latest approach to irritable bowel syndrome. The speaker will be Dr Kyle Staller, Director of Gastrointestinal Motility Laboratory, Harvard University, US.
Oral free papers and poster presentations are also included in the agenda and have increased this year, according to Dr Tham.
“We’ve increased the number of oral and poster presentations this year to highlight original research from our young, talented Irish investigators because the trainees and young investigators are the future of our society.
“I would encourage people to attend virtually; take the study day off and immerse themselves in the education,” he added.
Society
As well as organising a packed agenda for the summer meeting, the Society has been active in engaging with its international counterparts, said Dr Tham, such as the European Society for Gastrointestinal Endoscopy.
“We’re looking beyond Ireland and collaborating with other societies in Europe.”
This will be Dr Tham’s last meeting as President of the Society before he hands over the reins to incoming President, Prof Deirdre McNamara, at the end of this meeting.
“I would like to thank you and the Society for giving me the honour and privilege to be your President for the last
two years. It has truly been the highlight of my career. It has been a most challenging time with the pandemic, but what we as a Society have learned is to be able to adapt to changes. I look back with pride on what the Society has achieved over the past two years with the ‘Es’: Engaging with other societies and clinical services, collaborating with Europe, and educating our members,” he said.
Covid-19
The pandemic has had a devastating impact on the healthcare service and continues to present challenges for clinicians.
According to Dr Tham, the disease has resulted in a huge backlog of gastroenterology cases, both North and South of the border.
In April, more than 14,000 patients were waiting on an outpatient gastrointestinal appointment, according
to the National Treatment Purchase Fund, compared to about 12,000 in January 2020.
The resulting treatment delays will result in more delayed diagnoses, he said.
“On the whole island of Ireland our waiting lists are pretty terrible. There will be delayed diagnosis of cancer and patients, with serious benign conditions being detected later. That’s the side-effect of Covid on our patients.
“We’ve seen an increase in the referral rate since Covid and so with the increased referrals we are detecting more cancers as well. It’s hard to know what the impact would be not for Covid, but you need a huge data set for that.
I would anticipate that many years down the line, when they analyse the data, that cancer survival rates may dip. That’s speculation at the moment, but it makes sense if you detect cancers later that the prognosis would not be as good, but we don’t have the data yet to show that.”
modified-release mesalazine
Preview Conference THE MEDICAL INDEPENDENT | 31 MAY 2021 29
20424-Asacolon 1600mg Advertisement code 2021/8-240x165.indd 1 21/05/2021 14:21
Improve the efficacy cut the volume vs
PLENVU® PM/AM dosing was superior to MOVIPREP® in the per protocol population (successful overall cleansing 97.3% vs 92.2%, p=0.014)
Safety profile comparable to MOVIPREP® 1,4-6
Powder for Oral Solution
PEG 3350, Sodium Ascorbate, Sodium Sulfate, Ascorbic Acid, Sodium Chloride, and Potassium Chloride
This is an external link to a promotional website for PLENVU ® , intended for healthcare professionals only
IE PRESCRIBING INFORMATION: Plenvu powder for oral solution (Macrogol 3350 + Sodium ascorbate + Ascorbic acid + Sodium sulfate anhydrous + Potassium chloride + Sodium chloride)
Please refer to the full Summary of Product Characteristics (SmPC) before prescribing.
Presentation: Plenvu powder for oral solution is administered in two doses. Dose one is made up of 1 sachet containing: macrogol 3350 100g, sodium sulfate anhydrous 9g, sodium chloride 2g, potassium chloride 1g. Dose 2 is made up of 2 sachets (A and B). Sachet A contains: macrogol 3350 40g, sodium chloride 3.2g, potassium chloride 1.2g. Sachet B contains: sodium ascorbate 48.11g, ascorbic acid 7.54g.
Indication: For bowel cleansing in adults, prior to any procedure requiring a clean bowel.
Dosage and administration: For oral use. Adults and elderly: A course of treatment consists of two separate non-identical 500ml doses of Plenvu. At least 500ml of additional clear fluid must be taken with each dose. Treatment can be taken according to a two-day or one-day dosing schedule. Two-day dosing schedule: First dose taken the evening before the procedure. Second dose in the early morning of the day of the procedure. Morning only dosing schedule: Both doses taken the morning of the procedure. The two doses should be separated by a minimum of 1 hour. Day before dosing schedule: Both doses taken the evening before the procedure. The two doses should be separated by a minimum of 1 hour. No solid food should be taken from the start of the course of treatment until after the clinical procedure. Consumption of all fluids should be stopped at least 2 hours prior to a procedure under general anaesthesia or 1 hour prior to a procedure without general anaesthesia. Children: Not recommended for use in children below 18 years of age. Patients with renal or hepatic impairment: No special dosage adjustment is deemed necessary in patients with mild to moderate renal or hepatic impairment.
Patients should be advised to allow adequate time after bowel movements have subsided to travel to the clinical unit.
Contraindications: Hypersensitivity to the active substances or to any of the excipients, gastrointestinal obstruction or perforation, ileus, disorders of gastric emptying (gastroparesis, gastric retention), phenylketonuria, glucose-6-phosphate dehydrogenase deficiency, toxic megacolon.
Warnings and precautions: The fluid content of reconstituted Plenvu does not replace regular fluid intake. Adequate fluid intake must be maintained. As with other macrogol containing products, allergic reactions including rash, urticaria,

pruritus, angioedema and anaphylaxis are a possibility. Caution should be used with administration to frail or debilitated patients, in patients with impaired gag reflex, with the possibility of regurgitation or aspiration, or with diminished levels of consciousness, severe renal impairment, cardiac failure (grade III or IV of NYHA), those at risk of arrhythmia, dehydration or severe acute inflammatory bowel disease.
In debilitated fragile patients, patients with poor health, those with clinically significant renal impairment, arrhythmia and those at risk of electrolyte imbalance, the physician should consider performing a baseline and post-treatment electrolyte, renal function test and ECG as appropriate.
Any suspected dehydration should be corrected for before use of Plenvu.
There have been rare reports of serious arrhythmias including atrial fibrillation associated with the use of ionic osmotic laxatives for bowel preparation, predominantly in patients with underlying cardiac risk factors and electrolyte disturbance.
If patients develop any symptoms indicating arrhythmia or shifts of fluid/electrolytes during or after treatment, plasma electrolytes should be measured, ECG monitored and any abnormality treated appropriately.
If patients experience severe bloating, abdominal distension, or abdominal pain, administration should be slowed or temporarily discontinued until the symptoms subside.
Post-marketing cases of ischaemic colitis, including serious, have been reported in patients treated with macrogol for bowel preparation. Macrogol should be used with caution in patients with known risk factors for ischaemic colitis or in case of concomitant use of stimulant laxatives (such as bisacodyl or sodium picosulfate).
Patients presenting with sudden abdominal pain, rectal bleeding or other symptoms of ischaemic colitis should be evaluated promptly.
The sodium content, 458.5mmol (10.5g), should be taken into consideration for patients on a controlled sodium diet. The potassium content, 29.4mmol (1.1g), should be taken into consideration by patients with reduced kidney function or those on a controlled potassium diet.
Interactions: Medicinal products taken orally within one hour of starting colonic lavage with Plenvu may be flushed from the gastrointestinal tract unabsorbed. The therapeutic effect of drugs with a narrow therapeutic index or short half-life may be particularly affected.
Plenvu may result in a potential interactive effect if used with starch-based food thickeners. Macrogol ingredient counteracts the thickening effect of starch, effectively liquefying preparations that need to remain thick for people with swallowing problems.
6. MOVIPREP® Orange Summary of Product Characteristics. Available for Ireland from: www.medicines.ie.
Fertility, pregnancy and lactation: There are no data on the effects of Plenvu on fertility in humans. There were no effects on fertility in studies in male and female rats.
It is preferable to avoid the use of Plenvu during pregnancy. It is unknown whether Plenvu active ingredients/metabolites are excreted in human milk. A risk to the newborns/infants cannot be excluded. A decision must be made whether to discontinue breast-feeding or to abstain from Plenvu therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Effects on ability to drive and use machines: Plenvu has no influence on the ability to drive and use machines.
Undesirable effects: Diarrhoea is an expected outcome. Common: vomiting, nausea, dehydration. Uncommon: abdominal distension, anorectal discomfort, abdominal pain, drug hypersensitivity, headache, migraine, somnolence, thirst, fatigue, asthenia, chills, pains, aches, palpitation, sinus tachycardia, transient increase in blood pressure, hot flush, transient increase in liver enzymes, hypernatraemia, hypercalcaemia, hypophosphataemia, hypokalaemia, decreased bicarbonate, anion gap increased/ decreased, hyperosmolar state.
Refer to the SmPC for a full list and frequency of adverse events.
Legal category: Product subject to medical prescription which may be renewed
MA Number: PA 1336/005/001
MA Holder: Norgine B.V., Antonio Vivaldistraat 150, 1083HP Amsterdam, The Netherlands
Additional information is available on request or in the SmPC. For further information contact: Norgine Pharmaceuticals Limited, Norgine House, Moorhall Road, Harefield, Middlesex, United Kingdom UB9 6NS. Telephone: +44(0)1895 826606. Email: medinfo@norgine.com
Date of preparation: March 2021
Company reference: IE-GE-PLV-2100006
Healthcare professionals are asked to report any suspected adverse reactions via the HPRA Pharmacovigilance Website: www.hpra.ie. Adverse events should also be reported to Norgine Pharmaceuticals Ltd on:
Tel: +44 (0)1895 826 606

Email: medinfo@norgine.com
Available for Ireland from www.medicines.ie;
MOVIPREP®1
IE-GE-PLV-2100002 Date of preparation: April 2021 From Norgine, the company that brought you MOVIPREP ® (Macrogol 3350, sodium sulphate, ascorbic acid, sodium ascorbate and electrolytes) The first 1 litre PEG bowel preparation1-3 For more information please visit www.plenvu.co.uk References: 1. Bisschops R, et al. Endoscopy 2018; doi: 10.1055/a-0638-8125; 2. Schreiber S, et al. Endoscopy 2018; doi: 10.1055/a-0639-5070; 3. DeMicco MP, et al. Gastrointest Endosc 2018; doi: 10.1016/j.gie.2017.07.047; 4. PLENVU® Summary of Product Characteristics. Available for Ireland from www.medicines.ie; 5. MOVIPREP® Summary of Product Characteristics.
IE PRESCRIBING INFORMATION: Moviprep and Moviprep Orange (Macrogol 3350, sodium sulfate anhydrous, ascorbic acid, sodium ascorbate, sodium chloride and potassium chloride) PLEASE REFER TO THE FULL SUMMARY OF PRODUCT
CHARACTERISTICS (SmPC) BEFORE PRESCRIBING. Presentation: Powder for oral solution contained in two separate sachets, A and B. Sachet A contains macrogol 3350 100g; sodium sulfate anhydrous 7.5g; sodium chloride 2.691g and potassium chloride 1.015g. Sachet B contains ascorbic acid 4.7g and sodium ascorbate 5.9g. Uses: Bowel cleansing prior to any clinical procedure requiring a clean bowel. Dosage and administration: For oral use. Adults and Older People: A course of treatment consists of two litres of Moviprep / Moviprep Orange. A litre consists of one sachet A and one sachet B dissolved together in water to make one litre of solution. The reconstituted solution should be drunk over a period of one to two hours. This process should be repeated with a second litre to complete the course. A further litre of clear fluid is recommended during the course of treatment. This course of treatment can be taken either as divided or as single doses and timing is dependent on whether the clinical procedure is conducted with or without general anaesthesia as specified below: For procedures conducted under general anaesthesia:
1. Divided doses: one litre in the evening before and one litre in the early morning of the day of the clinical procedure. Ensure consumption of Moviprep / Moviprep Orange as well as any other clear fluids has finished at least two hours before the start of the clinical procedure. 2. Single dose: two litres in the evening before the clinical procedure or two litres in the morning of the clinical procedure. Ensure consumption of Moviprep / Moviprep Orange as well as any other clear fluids has finished at least two hours before the start of the clinical procedure. For procedures conducted without general anaesthesia: 1. Divided doses: one litre in the evening before and one litre in the early morning of the day of the clinical procedure. Ensure consumption of Moviprep / Moviprep Orange as well as any other clear fluids has finished at least one hour before the start of the clinical procedure.
2. Single dose: two litres in the evening before the clinical procedure or two litres in the morning of the clinical procedure. Ensure consumption of Moviprep / Moviprep Orange has finished at least two hours before the start of the clinical procedure. Ensure consumption of any clear fluids has finished at least one hour before the clinical procedure. Patients should be advised to allow for appropriate time to travel to the colonoscopy unit. No solid food should be taken from the start of the course of treatment until after the clinical procedure.
Children: Not recommended below 18 years of age. Contraindications: Known or suspected hypersensitivity to any of the ingredients, gastrointestinal obstruction or perforation, disorders of gastric emptying, ileus, phenylketonuria, glucose-6phosphate dehydrogenase deficiency, toxic megacolon which complicates very severe inflammatory conditions of the intestinal tract including Crohn’s disease and ulcerative colitis. Do not use in unconscious patients. Warnings and precautions: Diarrhoea is an expected effect. Administer with caution to fragile patients in poor health or patients with serious clinical impairment such as impaired gag reflex, or with a tendency to aspiration or regurgitation, impaired consciousness, severe renal insufficiency (creatinine clearance <30 mL/min), cardiac impairment (NYHA grade III or IV), those at risk of arrhythmia (e.g. those on treatment for cardiovascular disease or who have thyroid disease), dehydration, severe acute inflammatory bowel disease. Dehydration, if present, should be corrected before using Moviprep / Moviprep Orange. The reconstituted Moviprep / Moviprep Orange does not replace regular fluid intake and adequate fluid intake must be maintained. Semi-conscious patients or patients prone to aspiration or regurgitation should be closely monitored during administration, particularly if this is via a nasogastric route. If symptoms indicating arrhythmia or shifts of fluid or electrolytes occur, plasma electrolytes should be measured, ECG monitored and any abnormality treated appropriately. In debilitated fragile patients, patients with poor health, those with clinically significant renal impairment, arrhythmia and those at risk of electrolyte imbalance, the physician should consider performing a baseline and post-treatment electrolyte, renal function test and ECG as appropriate. The possibility of serious arrhythmias, predominantly in those with underlying cardiac risk factors and electrolyte disturbance cannot be ruled out. If patients experience symptoms which make it difficult to continue the preparation, they may slow down or temporarily stop consuming the solution and should consult their doctor. Moviprep Orange is not recommended for patients with glucose-galactose malabsorption. Post-marketing cases of ischaemic colitis, including serious, have been reported in patients treated with macrogol for bowel preparation. Macrogol should be used with caution in patients with known risk factors for ischaemic colitis or in case of concomitant use of stimulant laxatives (such as bisacodyl or sodium picosulfate). Patients presenting with sudden abdominal pain, rectal bleeding or other symptoms of ischaemic colitis should be evaluated promptly. Moviprep/Moviprep Orange contains 363.2 mmol (8.4g) of sodium per course of treatment (two litres) equivalent to 420% of the WHO recommended maximum daily intake of 2g of sodium for an adult. To be taken into consideration by patients on a controlled sodium diet. Only a proportion (up to 112.4 mmol (2.6g) per course of treatment) of sodium is absorbed. It also contains 28.4 mmol (1.1g) of potassium per two litres. To be taken into consideration in patients with reduced kidney function or patients on a controlled potassium diet. Interactions: Oral medication should not be taken within one hour of administration as it may be flushed from the GI tract and not absorbed. Moviprep / Moviprep Orange may result in a potential interactive effect if used with starch-based food thickeners. Macrogol ingredient counteracts the thickening effect of starch, effectively liquefying preparations that need to remain thick for people with swallowing problems. Fertility, pregnancy and lactation: There are no data on the effects on fertility. There are no data on the use in pregnancy or lactation so it should only be used if judged essential by the physician. Effects on ability to drive and use machines: No influence on the ability to drive and use machines Side Effects: Very common: abdominal pain, nausea, abdominal distension, anal discomfort, malaise and pyrexia. Common: sleep disorder, dizziness, headache, vomiting, dyspepsia, rigors, thirst and hunger. Uncommon: dysphagia, abnormal liver function tests and discomfort. Not known: allergic reaction including anaphylactic reaction, dyspnoea and allergic skin reactions including angioedema, urticaria, pruritus, rash, erythema; electrolyte disturbances including blood bicarbonate decreased, hyper and hypocalcaemia, hypophosphataemia, hypokalaemia and hyponatremia and changes in the blood chloride levels; dehydration; convulsions associated with severe hyponatraemia; transient increase in blood pressure; arrhythmia, palpitations; flatulence and retching. Refer to the SmPC for a full list and frequency of adverse events. IRELAND Pack sizes: Lemon- or orange-flavoured powder in sachets, 1 treatment pack (2 x sachet A + 2 x sachet B). Legal category: Product subject to medical prescription which may be renewed. MA Number: PA 1336/001/001 (Moviprep); PA 1336/001/002 (Moviprep Orange). MA Holder: Norgine
BV, Antonio Vivaldistraat 150, 1083 HP Amsterdam, The Netherlands. Additional information is available on request or in the SmPC. For further information contact: Norgine Pharmaceuticals Limited, Norgine House, Moorhall Road, Harefield, Middlesex, UB9 6NS, UK. Tel: +44(0)1895 826606. Email: medinfo@norgine.com Date of preparation: April 2021 Company reference: IE-GE-MPR-2100001
Ireland
Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance, Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
Adverse events should also be reported to Norgine Pharmaceuticals Ltd on: Tel: +44 (0)1895 826 606. Email: medinfo@norgine.com
Irish Society of Gastroenterology, Summer Meeting, virtual programme, 18 June 2021

PLENVU, MOVIPREP, NORGINE and the sail logo are registered trademarks of the Norgine group of companies.
16.30
in symptomatic disease
Prof Ramesh Arasaradnam
Consultant Gastroenterologist, University of Warwick, UK
The latest approach to irritable bowel syndrome
Kyle Staller, MD
Director of Gastrointestinal Motility Laboratory, Harvard University, US
16.55
Close of meeting
(Commercial video)
09.30 Commercial video 09.50 Oral Free Papers 1-4 10.30 Early gastric cancers and pre-cancer detection and management
Mario Dinis-Ribeiro Consultant Gastroenterologist, Head of Gastroenterology Dept, Portuguese Oncology, Institute of Porto 10.55 Gastric surgery – The future
Paul Carroll Oesophagogastric and General Surgeon, Galway University Hospital 11.20 Commercial video 11.30 Gastrointestinal consequences of pelvic radiation disease
Jervoise Andreyev Consultant Gastroenterologist and Hon Professor, University of Nottingham, UK 11.55 Preventing post-operative recurrence of Crohn’s disease
David Kevans Consultant Gastroenterologist, St James’s Hospital, Dublin 12.20 Commercial video 12.30 Top 10 posters 13.55 Oral Free Papers 5-8 14.30 Haemochromatosis – what’s new?
Prof
Mr
Prof
Dr
Hepatologist,
James’s
Dublin 14.55 Commercial video 15.05 Managing ascites in cirrhosis
15.30 Oesophageal dysmotility
15.55 Commercial video 16.05
Prof Susanne Norris Consultant
St
Hospital,
Prof Guru Aithal Professor of Hepatology and Head of Division for Digestive Diseases, University of Nottingham, UK
Eamonn Quigley, MD Past President of WGO, Chief of Gastroenterology and Hepatology at Houston Methodist Hospital, Texas, US
Using FIT
Agenda Conference
31 THE MEDICAL INDEPENDENT | 31 MAY 2021
Getting it right with chronic urticaria
Attendees at UCD’s Charles Institute Seminar Series recently heard a presentation by Prof Markus Magerl on clinical considerations in the pathophysiology of chronic urticaria


The Charles Institute, Ireland’s national dermatology research and education centre, hosts a range of guest speakers who cover a variety of topics ranging from skin cancer to psoriasis, among others. The series, which is sponsored by RELIFE (part of the A.Menarini group), is designed to provide expert advice from a range of distinguished national and international experts in their respective fields and is chaired by Prof Desmond Tobin, Full Professor of Dermatological Science at UCD School of Medicine and Director of the Charles Institute of Dermatology. The seminars are broadcast to attendees with a special interest in dermatology and cutaneous science in other locations, who access the talks remotely via an audio-visual link.
Attendees heard a presentation from Prof Markus Magerl, Professor of Dermatology at the Charité Universitätsmedizin in Berlin, Germany, on the topic ‘Pathophysiology of Chronic Urticaria’. Prof Magerl provided an outline of chronic urticaria, explaining that it is a common disease that features recurrent itchy wheals, angioedema, or both, in response to either a specific stimulus (chronic inducible urticaria, CINDU), however in most instances it can occur spontaneously (ie, chronic spontaneous urticaria, or CSU).
Quality of life can be dramatically impaired in patients with the condition, and in order to provide an optimal management of their health, physicians need to understand its pathophysiology, the potential for differential diagnoses, and current treatment guidelines.
For most patients, CSU has two distinct immune-mediated mechanisms, he explained — type I (ie, autoallergy); and type IIb autoimmunity. In type I autoallergy patients, IgE autoantibodies to autoallergens are found, for example IgE anti-TPO or anti-IL-24. In type IIb, IgG autoantibodies target activating Mast Cell (MC) receptors, for example FcεRI). As underlying autoimmune mechanisms cannot be treated causally, symptomatic therapy is required and Prof Magerl stressed the need to first rule out differential diagnoses, such as autoinflammatory syndromes, urticaria vasculitis or bradykinin-mediated angioedema.
He provided a brief overview of the standard treatment in urticaria, which comprises a second-generation antihistamine in standard or higher doses. He told the seminar that many patients, however, still suffer, despite having received the recommended antihistamine treatment. In these patients, the only currently licensed drug is omalizumab, a monoclonal antibody against IgE. Some patients with type I autoallergy respond exceptionally well to omalizumab, he said, while type IIb autoimmune patients take a longer time to respond
or are refractory.
However, regardless of underlying pathophysiology, diagnosis and a proper treatment allows physicians to effectively treat most patients, explained Prof Magerl. New treatment alternatives — including novel anti-IgE antibodies, ie, antibodies inhibiting any mast cell activation — are currently in clinical trials and have shown promise, offering the hope to soon treat all CSU patients effectively, he told the attendees.
Chronic disease
Prof Magerl presented case studies on CSU, which is the most common form of urticaria, and told the seminar: “Many patients display wheals on their skin; many patients have angioedema but most patients suffer from both wheals and angioedema, but not always at the same time. One day, the patients may be suffering from wheals, and another day they could be suffering from angioedema,” he explained. “Urticaria is a chronic disease and once it becomes chronic, it progresses to being very chronic. Using registry data, we see that [in 50 per cent of patients] the median time a patient suffers with urticaria is between two and three years, so it is self-limiting. However, in the other 50 per cent, they suffer for three years or more and some patients even suffer with chronic spontaneous urticaria for decades, and this can seriously affect their quality of life.”
agnosis in patients who display only wheals is urticaria vasculitis and this is suspected when the duration of a single wheal is longer than 24 hours, Prof Magerl explained, and a skin biopsy should be taken to rule out urticaria vasculitis. However, in patients who only display angioedema, other differential diagnoses should be considered and these patients should be first asked if they are taking an ACE inhibitor, which can then be discontinued to see if that affects the condition. Hereditary angioedema is another rare differential diagnosis, and should be suspected if the symptoms appear at a young age and there is a family history, as well as painful internal abdominal swelling.
Pathophysiology
He provided a synopsis of the pathophysiology of urticaria and showed evidence that infections or food intolerances can play a role as disease modulators in CSU, but not as underlying cause.
is plenty of evidence that more antihistamines results in more responders,” said Prof Magerl.
Prof Magerl provided an overview of the drugs currently in development for the treatment of urticaria and explained that over recent years, therapies are being developed specifically for urticaria. In this regard, he described how it has been found that the mast cell is involved in ‘cross-talk’ with other cells in the immune system, for example eosinophils, basophils, T-cells and B-cells, among others. “There are single reports that treatment of these cross-talk partners can be beneficial in patients with urticaria,” he said.
Prof Magerl summarised his presentation by saying: “CSU has a deep impact on patients’ quality of life and needs to be treated effectively. We have different treatments, such as antihistamines, omalizumab, cyclosporine, and there are a lot of new drugs in development.
“We differentiate two types of underlying causes as a pathomechanism in chronic spontaneous urticaria,” he continued. “On the one hand, we have type I autoallergy, and we also see type IIb autoimmunity in patients with this condition, and these patients differ in their clinical characteristics and in their response to drugs, and that’s fascinating. We are continuing to reveal how this condition works and how we can classify the remaining patients who currently do not fit into the categories of allergy or immunity.”
Wheals
For dermatologists, urticaria is a straightforward diagnosis, as there is no other skin disease that is exactly like it, said Prof Magerl. “However, a diagnosis can be difficult in rare cases, especially when patients only display wheals or only angioedema. If patients only have wheals, we have to check for autoinflammatory diseases,” he told the seminar.
“We ask these patients about recurrent unexplained fever, joint or bone pain or malaise, and if patients answer ‘yes’ to one of these questions, we check for autoinflammatory disease, we check for CRP and serum amyloid, S100 [protein] and interleukin 1 and 6.”
Another potential for differential di-
“Through our work in recent years, we now have a completely different working hypothesis of the pathophysiology of urticaria,” said Prof Magerl. “We are convinced that urticaria is still working via mast cells and still working via histamine, however… we found that IgE is responsible for a large proportion of urticaria cases. We have seen that many urticaria patients have a thyroid disease and 15-to-30 per cent of these patients develop or display antibodies in the blood. Another observation we have made is that patients with chronic spontaneous urticaria have increased IgE total levels that are much higher than healthy controls.” Further research has shown a propensity for a type I autoimmunity or autoallergy in these patients, he added.
Discussing treatment options, Prof Magerl told the seminar that second-generation non-sedating antihistamines are still the first-line option for CSU. A minority of patients respond well to a single dose, so the guidelines recommend starting with a single dose and if there is inadequate response after two-to-four weeks, the dose should be up-titrated and can be increased up to four-fold, he said [Note: this may constitute off-licensed dosing for some products]. “There
During a discussion and Q&A session following Prof Magerl’s presentation, Prof Tobin raised a point regarding the appearance of wheals in cases of CSU. “They are defined as wheals because they are circumscribed and have defined borders, but in some of the case studies you presented, they almost appeared to merge and become more confluent. Is there anything known about what regulates them, as this is also a fascination in auto-inflammatory conditions like alopecia areata? So in other words, if wheals increase in number, do they generally stay separate, or is the surface area increased because they merge?”
Prof Magerl responded: “The general appearance of a wheal is very monomorphic — it always looks elevated, like a nettle sting reaction which is between reddish and whitish and is elevated and itchy. However, how it develops can be polymorphic, but single wheals can increase [in size] and become confluent. That’s typical behaviour but it’s impossible to predict how the course of urticaria will develop over the following minutes or hours [after activation]. We still have no in vivo trigger of mast cells where we could activate mast cells and that’s why we use cold urticaria as a disease model.”
RELIFE has had no input into the content of this article or series of seminars

Clinical Dermatology THE MEDICAL INDEPENDENT | 31 MAY 2021 32
Article and series in association with UCD CHARLES INSTITUTE SEMINAR SERIES
Prof Markus Magerl
Through our work in recent years, we now have a completely different working hypothesis of the pathophysiology of urticaria
DRY & ROUGH SKIN UNDER CONTROL, FROM HEAD TO TOE
















Clinically proven for use all over the body with moisturising and keratolytic formulations even for plaques associated with psoriasis.















Let your skin benefit from U-LifeTM, the one-stop solution with moisturising and keratolytic action for the management of dry, very dry, and chapped skin in specific areas of the body. U-LifeTM creams and anhydrous pastes are formulated with di erent urea percentages plus other specific ingredients to meet your skin’s needs and target specific areas (scalp, face, body, hands and feet).

































RELIFE ™ MY SKIN SAYS HOW I FEEL.
















































































Date of Item Dec 2020 IE20071 IR-REL-120-2020
WWW.RELIFE.IE Ulife 370 x 270 range advert.indd 1 18/03/2021 10:38
SPORTS QUIZ WIN €50 31 May 2021
Q1 Which racecourse will host the 2021 Irish Derby next month?
Q2 Who won the US PGA championship last week?
Q3 Which team won the 2021 European Challenge Cup?
Q4 St Patrick’s Athletic play their home games in which stadium?
Q5 The British and Irish Lions will play which country in Murrayfield, prior to heading to South Africa?
Q6 Who will Tyson Fury defend his WBC World Heavyweight title against this coming July?
CROSSWORD COMPETITION
31 May 2021
The winner of the 10 May 2021 Sporting Quiz Competition is Dr Marion Glasheen, Waterford
The winner of the 10 May 2021 Crossword is Dr Geraldine Brosnan, Dublin
Q1 Who is the 2021 World Snooker Champion? A: Mark Selby
Q2 Who finished the Irish National Hunt season as top jockey? A: Paul Townend
Q3 Which city will host this year’s Champions League final? A: Istanbul
Q4 Who will face off in this year’s Heineken Champions Cup final later this month? A: La Rochelle v Toulouse
Q5 Who won this year’s Portuguese Grand Prix? A: Lewis Hamilton
Q6 Cycling’s Giro d’Italia commenced last week. What colour jersey does the leader/winner wear? A: Pink
2 Prepare and issue for sale (7)
3 Given generously (7)
4 Medical treatment centre (6)
5 Taut (5)
6 Command (5)
7 Milieu (11)
8 Instantly (11)
14 Learner (7)
15 Act of turning up (7)
17 Step down from a job (6)
19 Quantitative relation between two amounts (5)
20 Distinguishing characteristic (5)
Life Mindo Quizzes THE MEDICAL INDEPENDENT | 31 MAY 2021 34 Post your answers to: Mindo Quizzes, The Medical Independent, Greencross Publishing Ltd, Top Floor, 111 Rathmines Road Lr, Dublin 6. Closing date for entries is 10 June 2021 Solution to Crossword Competition
Answers to Sports Quiz Competition Solution to Sudoku P O S S I B L Y L E S S A Q N I N T S T U F F Q U A R T E R S A L U I A R U I N H E R I T P R E T E N D U E E H N R G Y S C S U R G E R Y S Q U E E Z E I M I M T C E A C O M M E N T A R R O W A I L N G A L A T E S E V E R E L Y 3 4 8 1 5 7 2 6 9 9 5 2 6 8 4 3 1 7 1 7 6 3 2 9 5 4 8 5 9 1 7 3 6 8 2 4 2 6 3 9 4 8 1 7 5 7 8 4 5 1 2 9 3 6 8 2 9 4 6 1 7 5 3 6 3 7 2 9 5 4 8 1 4 1 5 8 7 3 6 9 2 4 1 3 7 9 6 8 9 2 4 1 3 9 6 4 5 6 8 7 9 1 8 6 5 4 1 SUDOKU SCRIBBLE BOX 10 MAY 2021 ANSWERS, SOLUTIONS, AND WINNERS WIN €50 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Across 1 - Piece of software (11) 9 - Living thing (5) 10 - Water barrier (3) 11 - Innate worth (5) 12 - One more than two (5) 13 - Investigate (8) 16 - Standards (8) 18 - Worthiness (5) 21 - Propel forwards (5) 22 - Ten (anag) (3) 23 - Continuing in existence (5) 24 - Luckily (11) Down 2 - Prepare and issue for sale (7) 3 - Given generously (7) 4 - Medical treatment centre (6) 5 - Taut (5) 6 - Command (5) 7 - Milieu (11) 8 - Instantly (11) 14 - Learner (7) 15 - Act of turning up (7) 17 - Step down from a job (6) 19 - Quantitative relation between two amounts 20 -
Piece of
(11)
Living
Water barrier (3)
Innate
(5)
One more than
Investigate
Standards
Worthiness (5)
Propel
(5)
Ten
(3)
in existence (5)
Luckily (11)
Distinguishing characteristic (5) ACROSS 1
software
9
thing (5) 10
11
worth
12
two (5) 13
(8) 16
(8) 18
21
forwards
22
(anag)
23 Continuing
24
DOWN
On the complexity of calories
Can you remember what a calorie is? Let me see. My recollection is that it’s the energy required to raise the temperature of a litre of water by... er... um... a certain amount?
So, clearly I had to check and it turns out that it’s the amount of energy needed to raise the temperature of 1g of water by 1ºC or, to be precise, 4.1868 joules.
Well, I’m sure you’re glad we cleared that up.
The ‘calories in, calories out’ theory has been with us, in developing forms, since Antoine-Laurent de Lavoisier built the first calorimeter shortly before losing his head – literally – during the French Revolution. The American physiologist Wilbur Atwater developed the modern concept in the closing years of the 19th Century, burning food to see how much energy it contains.
Indeed, when using student volunteers in the course of his metabolic studies, he would set fire to their faeces. Where other scientists would have perhaps tried to find a way around this challenging and malodorous business, Atwater didn’t flinch. Although, I should add that I can’t find any reference to how he actually managed the incineration process.
His motivation was not about calorific restriction. Instead, he was concerned that the poor were not getting enough calories in their diet and he wanted to be able to make scientific recommendations to help improve their lot.
Anyway, it’s a curious thought that ‘calories in, calories out’ is still the mantra well over a century after Atwater refined it. It underpins the ‘eat less, move more’ school of weight control thinking and has resulted in the dietary obsession that was kick-started by the US Senate in 1977 when it recommended a low fat, low cholesterol diet for all. The fat was, of course, replaced by sugar and salt and global obesity trebled between 1975 and 2016.
At this stage it is estimated that 1.9 billion people are overweight. It comes as no surprise that the incidence of type 2 diabetes has more than doubled over the past 40 years.
Incidentally, the most obese country in the world, with 74.6 per cent of the population overweight, is American Samoa. The least obese is Vietnam with a shade over 2 per cent. The US weighs in at 36.2 per cent, the UK at 27.8 per cent, Ireland at 25.3 per cent, and food-loving France at 21 per cent.
Of course, the demonisation of fat was an obsession of another American scientist, Ancel Keys, and the object of his socalled lipid hypothesis about which I’ve written here before. The process, however, was clandestinely encouraged by the US sugar industry and in 2016 a researcher at the University of California found the smoking gun.

Documents uncovered by Prof Stanton Glantz of UC San Francisco showed that in 1967 the Sugar Research Foundation, a sugar industry body now known as the Sugar Association, paid Harvard scientists to publish cherry-picked studies that minimised links between sugar consump -
tion and heart disease, while pointing the finger at dietary fat. The publication that unleashed this on the world was no less than the New England Journal of Medicine (NEJM). The NEJM has required disclosure of financial assistance only since 1984.
“They were able to derail the discussion of sugar for decades,” Glantz told The New York Times .
fectionery industry was funding research with an almost comical theme: To suggest that children who eat sweets weigh less than those who don’t. One is left wondering where they found a cohort of the latter, but never mind.
The Sugar Association, when questioned about the 1967 outrage, admitted that it should have been more transparent, but added that industry-funded research had shown that sugar does not have a unique role in heart disease. My emphasis.
Speaking of diet and calories, I came across an interesting snippet the other day: It seems that chopping and cooking food makes calories more easily accessible during digestion. Richard Wrangham, a primatologist at – once again – Harvard, suggests that the evolution of humankind needed the development of cooking. I shall remember that when I’m next rattling the pans.
None of the Harvard scientists are still alive, but one of them, David Mark Hegsted went on to be head of nutrition at the US Department of Agriculture where he drafted the 1977 government dietary guidelines.

It appears that the sugar lobby is still trying very hard to shape the narrative on diet. It was reported in 2015, again by The New York Times , that Coca-Cola had provided millions of dollars in funding to scientists who “sought to play down the link between sugar and obesity”. Shortly afterwards it was revealed that the US con-
I also learned more about a subject I mentioned here before: Resistant starch. The theory is that, say bread or pasta, that has been cooked, cooled, and reheated (like toast or microwaved leftover tagliatelle) has its glycaemic index (GI) lowered. I now read that scientists in Sri Lanka have found that the GI of rice that is cooked, anointed with coconut oil, cooled and reheated is halved. I can’t find a reference to an in vivo study, but it’s encouraging for those of us who like an occasional carb fest.
This puts me in mind of the joys of toasted sourdough with maybe some goose or pork rillettes and I have the perfect wine to accompany such an indulgence. It delivers a further bonus in having very, very little residual sugar. This is also an opportunity to remind readers that Whelehans Wines, in what used to be The Silver Tassie in Loughlinstown, is an oenophile’s dream.
As summer hesitantly approaches my thoughts turn to the red wines of the Loire and their showcasing of the Cabernet Franc grape, their vibrant acidity, vivid fruit, and grippy tannins.
Food wines, in every sense and Domaine Filliatreau Saumur-Champigny ‘Lena Filliatreau’ 2018 (€17, sale price) is a juicily delicious example at a very keen price.
It has the acidity to tackle the fat that we no longer demonise and I suspect this would be a lovely foil to the richness of slow-cooked pork belly or shoulder of lamb.

Food & Drink Life THE MEDICAL INDEPENDENT | 31 MAY 2021 35
Read more at www.mindo.ie @tomdoorley
TOM DOORLEY
It seems that chopping and cooking food makes calories more easily accessible during digestion
HSE Covid-19 media briefings, Dr Steeven’s Hospital, Dublin






THE MEDICAL INDEPENDENT | 31 MAY 2021 36 Life Gallery
Pictured L-R: Dr Colm Henry, Chief Clinical Officer, HSE; and Mr Damien McCallion, HSE National Lead, Vaccination Programme, HSE
HSE CEO Mr Paul Reid
Ms Anne O’Connor, Chief Operations Officer, HSE HSE CEO Mr Paul Reid; Ms Anne O’Connor, Chief Operations Officer, HSE; and Dr Colm Henry, Chief Clinical Officer, HSE
Dr Greg Martin, Public Health Specialist, HSE
Dr Colm Henry, Chief Clinical Officer, HSE
RCPI PUBLISHES PAPER ON BREASTFEEDING
The RCPI has called for greater support for breastfeeding families, better education for the public and for healthcare professionals, and improvements in hospital facilities to support breastfeeding.
A paper from the Faculty of Paediatrics, Faculty of Public Health, and the Institute of Obstetricians and Gynaecologists of the RCPI highlights that despite improvements over the last 15 years, breastfeeding rates in Ireland remain below international targets. Cultural norms, knowledge gaps among healthcare professionals and lack of practical support impact on these rates.
Spokesperson Dr Anne Doolan, Consultant Neonatologist, and International Board Certified Lactation Consultant (IBCLC), and one of the lead authors of the paper, said: “We know that breastfeeding provides optimal nutrition for infant’s growth and development and that it has the potential to improve health on a nationwide scale. We have national policy, which contributes to normalising breastfeeding, but barriers still exist.
“It is difficult for many to access specialist support when needed. We have very limited numbers of publicly funded lactation consultants. Such support can make a huge difference in getting breastfeeding off to a good start.”
“Public health nurses too play an integral role as they meet mothers and infants shortly after hospital discharge and can identify breastfeeding challenges, establish individualised care plans or refer to an IBCLC if necessary.
“This role can be strengthened with more public health nurse resources focused on child health and wellbeing services, as per Sláintcare and the National Health Childhood
Programme.”
Dr Meredith Kinoshita, also one of the paper’s lead authors and Specialist Registrar in Paediatrics, noted that mothers report receiving conflicting information about breastfeeding from health professionals, which leads to uncertainty and undermines confidence.
Dr Kinoshita said: “Healthcare professionals should have the appropriate education and skillset to support breastfeeding, with consistent information and continuity between services in hospitals and the community. Clinical exposure is key in this regard.”

“Hospital facilities should also support breastfeeding, for example when breastfeeding mothers are admitted as inpatients.
Rooming-in facilities and equipment and facilities for expressing and storing breastmilk should be provided in those instances. The establishment of a donor milk service for the Republic of Ireland would also be welcomed.”
Dr Louise Kyne, Dean of the Faculty of Paediatrics, said: “Paediatricians and obstetricians in particular have an influential role in supporting the initiation and continuation of breastfeeding as they meet infants and their families often in the perinatal period.
“RCPI as the postgraduate specialist training body for these specialities is committed to ensuring that its training curricula and continuing professional development, equip doctors with the appropriate knowledge to support breastfeeding.”
The paper also affirms RCPI’s commitment to hosting all meetings, conferences, and study days free from sponsorship from breastmilk substitutes in accordance with the World Health Organisation code of marketing of breastmilk substitutes.
ASTELLAS’S XTANDI (ENZALUTAMIDE) APPROVED BY EUROPEAN COMMISSION FOR MEN WITH METASTATIC HORMONESENSITIVE PROSTATE CANCER
Astellas Pharma has announced the European Commission (EC) has approved an additional indication for the oral once-daily therapy Xtandi (enzalutamide) for adult men with metastatic hormone-sensitive prostate
cancer (mHSPC, also known as metastatic castration-sensitive prostate cancer or mCSPC). Men diagnosed with mHSPC tend to have a poor prognosis, with a median survival of approximately three-to-four years, under-
scoring the need for new treatment options.
With this indication, enzalutamide is now the only oral treatment approved by the EC to treat three distinct types of advanced prostate cancer – non-metastatic and metastatic castration-resistant prostate cancer (CRPC) and mHSPC. The EC approval is based on results from the pivotal phase 3 ARCHES trial, which evaluated enzalutamide in men with mHSPC.
“Metastatic hormone-sensitive prostate cancer patients have limited options and, unfortunately, there is a poor prognosis for many men,” said Prof Andrew Armstrong, Professor of Medicine, Surgery, Pharmacology and Cancer Biology, Director of Research in the Duke Cancer Institute’s Center for Prostate and Urologic Cancers, US, and lead investigator of ARCHES.
“The research supporting this approval provides clinical evidence showing how enzalutamide can help improve outcomes for men with mHSPC, which gives healthcare professionals in Europe the option to offer the treatment across the advanced prostate cancer disease continuum.”
Data from the ARCHES trial showed enzalutamide plus androgen deprivation therapy (ADT) significantly reduced the risk of radiographic progression or death by 61 per cent versus placebo plus ADT in men with mHSPC (n=1,150; hazard ratio [HR]=0.39 [95 per cent
confidence interval (CI): 0.30-0.50]; P<0.0001).
“Enzalutamide has been an established standard of care for men with advanced prostate cancer and has been prescribed to more than 610,000 patients worldwide since it was first approved in 2012,” said Dr Andrew Krivoshik, Senior Vice President and Global Therapeutic Area Head, Oncology Development, Astellas.
“This new indication for enzalutamide provides men with mHSPC a much-needed, additional therapy option earlier in their treatment journey. We look forward to working with health authorities across Europe to ensure men with mHSPC have access to enzalutamide as soon as possible.”
The safety analyses of the ARCHES trial appear consistent with the safety profile of enzalutamide in previous clinical trials in CRPC. In ARCHES, grade 3 or greater adverse events (AEs) (defined as severe/disabling or life-threatening) were similar for patients receiving both enzalutamide plus ADT and those who received placebo plus ADT (24.3 per cent vs. 25.6 per cent).
The EC marketing authorisation for enzalutamide in men with mHSPC is applicable to European Union member countries, and is also valid in Iceland, Norway and Liechtenstein.
This approval will have no impact on the financial forecasts of the current fiscal year ending 31 March 2022.
NUI GALWAY TO EXPAND CLINICAL TRIALS FOR DIABETES AND CHRONIC DISEASE
Professor in the College of Medicine, Nursing and Health Sciences at NUI Galway, is to lead the Diabetes Collaborative Clinical Trial Network which will work on an all-island basis.
“This investment is hugely significant for patients. Diabetes is one of the most common chronic diseases in Ireland and the number of people affected by it is increasing at an alarming rate alongside the increase in obesity rates,” Prof Dunne said.
“The aim of all the co-applicants and collaborators who have worked on this project is to ensure that people with diabetes across the island of Ireland have access to high quality clinical trials regardless of where they live.
“Through trials in new medicines and technologies we can improve health and reduce disease burden for patients with diabetes.”
Prof Dunne outlined some initial areas of focus for the Diabetes Collaborative Clinical Trial Network, includ-
ing diabetes in pregnancy, technologies, foot disease, advanced therapies, and behavioural change.
Prof Andrew Murphy, GP in Turloughmore Medical Centre, Co Galway and Professor of General Practice at NUI Galway, is to lead the Primary Care Clinical Trials Network.
“Our aim is simply to produce high-quality clinical evidence, which improves patient outcomes in primary care, where the vast bulk of healthcare is provided,” Prof Murphy said.
“Our high-level strategy prioritises the conduct of trials in chronic disease management, multi-morbidity where patients have two or more diseases, and infectious diseases. Over the past five years we have recruited almost 4,000 patients and had 20 registered trials, working with the Royal College of Surgeons in Ireland, the Irish College of General Practitioners, and other universities in Ireland and Europe. Our aim is to bring trials to hundreds more patients.”
SIMS IVF STRENGTHENS MEDICAL TEAM WITH NEW APPOINTMENTS
Sims IVF Group has announced the appointment of fertility experts to its clinics in Dublin and Cork. Dr Moses Batwala has been appointed as Clinical Director of the Cork Clinic, and Dr Karlo Tomicic, an obstetrician and gynaecology specialist, will be based at the Clonskeagh clinic in Dublin. Sims IVF Group said it now has 11 fertility experts working across its three clinics in Ireland.
Oxford’s Institute of Reproductive Sciences, and completed a MSc by research in quality-of-life of fertility patients. Dr Batwala graduated from the University of Leeds in medicine and surgery with honours in biochemistry in 2001.
People with diabetes and patients with chronic disease and those who use primary care are set to benefit from the expansion of clinical trials at NUI Galway.

The Health Research Board (HRB) has announced the Primary Care Clinical Trials Network and the Diabetes Collaborative Clinical Trial Network are to be supported at the uni-
versity.
The NUI Galway networks are two of six projects that have been selected nationwide following a rigorous application process and adjudication by an international panel of experts.
Prof Fidelma Dunne, Consultant Endocrinologist in University Hospital Galway and Saolta University Health Care Group and
Dr Batwala joins the Sims IVF team from IVF London, where he served as Medical Director. Prior to this, he held the position of Lead Fertility Consultant in Evewell Clinic and Create Fertility in London. He has a specialist interest in mild stimulation, treating women with low-ovarian reserve, and pre-implantation genetic testing (PGT).
From 2014 to 2016, he was a Senior Clinical Research Fellow at the University of

Dr Tomicic joins the Sims IVF team from University Hospital Centre Zagreb, Croatia, where he worked in the Department of Gynaecology and Obstetrics for nearly 10 years. During this time, he worked in endoscopic surgery involving laparoscopic and hysteroscopy procedures for treatment of uterine polyps, fibroids, ovarian cysts, and endometriosis. In 2015, he began working in the fertility unit combining surgery with assisted reproductive treatments.
Dr Tomicic graduated from the School of Medicine in University of Zagreb in 2005.
RXDX Product Focus THE MEDICAL INDEPENDENT |31 MAY 2021 37
Dr Moses Batwala
Prof Fidelma Dunne and Prof Andrew Murphy
Dr Karlo Tomicic
GP REQUIRED
Custom House Square Medical Centre is seeking a full-time salaried general practitioner to join a young(ish) dynamic team. The remuneration package is best in class and the working hours and conditions are most attractive.
Have a gander at our website www.custommedical.ie and if interested forward your CV to practicemanager@custommedical.ie
For informal enquiries please phone Dr Peter Killeen at 087 131 4139
GP REQUIRED
Rush Co Dublin
Share running of a single practice with a view to succession, in 2-to-3 years.
Public and private patients.
Socrates computer software. Tel – 086 861 0692
GP POSITION AVAILABLE
GP position available in Galway.
Seapoint Medical Centre located in Barna, Co Galway is a thriving 3 GP practice. We require an established compassionate, friendly, and energetic Assistant General Practitioner to join our team in a permanent capacity. Special interest in women’s health among your other interests would be an advantage. The successful GP will be required for 6 sessions per week.
Our practice is fully computerised using Socrates software and is appropriately supported by nursing, admin, and management. We have an attractive remuneration package for the suitable GP and some negotiable terms and conditions. Learn more about us at www.seapointmedical.com and express your interest or inquire further to Ronan at ronan@seapointmedical.com
or 086 419 2470
GP LOCUM REQUIRED
Short-term/long-term GP locum needed for Skibbereen Medical Centre in beautiful West Cork. Supportive and friendly practice. Excellent administrative and nursing staff. Email enquiries and CV to westcorkmedical@gmail.com
LOCUM REQUIRED
Locum GP required in North Inishowen area to cover leave for months of July, August, and September.
Group practice/fully computerised, full ancillary staff in a purpose-built primary care centre.
For further information on this position contact Dr Seamus Kelly via email skelly@millbraesurgery.ie
Millbrae Surgery, Carndonagh, Co Donegal
MEDICAL GP SURGERY EQUIPMENT FOR SALE
Entire contents of two treatment rooms, reception area, fridges, autoclave, etc, from closing down sale in Galway area.
Contact for list etc anseomra@gmail.com
Phone 085 725 4566
MEDICAL CENTRE AVAILABLE
Seafront Medical Centre available to rent from 1st July, Main St, Blackrock, Co Louth.
Existing medical practice in this building since 1989. Superb location, prestigious building, at bus stop, free parking. Three doctor’s consulting rooms, nurses room, receptionist and secretarial area, fine reception area and back office and toilets. All rooms have plenty of natural light and windows. Terrific opportunity for enthusiastic young doctors to set up practice in under-doctored population in sought after area in perfect location. Contact rockdoc89@gmail.com or 086 829 4445 for further information
GP LOCUM REQUIRED
We are looking for GP locum cover for the following weeks. 5th July until 27th August (8 weeks)
Location: Inishowen Medical Clonmany and Buncrana (mixed urban and rural practice).
Work: 8-9 sessions per week as part of a multi-doctor team. No out-of-hours requirements.
Practice fully computerised (HPM) and practice offering a very generous pay package.
Contact mmcgowanardaravan@gmail.com for further information
GP REQUIRED
GP required in Killarney. Six sessions per week. Immediate start. Modern surgery. Sessions flexible. Rapid progress to own GMS list.
For more information or enquiries contact Laura at info@killarneygp.ie or 087 990 1108
CLINIC CONSULTING ROOM AVAILABLE
Looking for a consulting room in the Galway Clinic to rent on a sessional basis? 1-8 sessions available weekly in a quiet, furnished ground floor suite.
Also, if you need a separate office full-time for your medical secretary or as a clinical room for a nurse or physiotherapist, there is a smaller office available to let full-time in the same suite.
Please phone 086 804 9891 for further information
Classifieds & Recruitment THE MEDICAL INDEPENDENT | 31 MAY 2021 38
MEDICAL SECRETARY REQUIRED
Experienced medical secretary required for part-time post in GP practice in Fethard, Co Tipperary.
Previous experience of working with HealthOne software and good knowledge of GMS system returns is essential.

Contact Dr Jaco Oosthuysen 087 692 8946 DrJacoOosthuysen@gmail.com
PRACTICE NURSE REQUIRED
Friendly group practice in South West Dublin seeking to recruit a permanent part-time practice nurse to join our team. Experience in practice nursing is desirable, but not essential. Suitable candidates from various nursing backgrounds will be considered. Job training offered, to build necessary skill set, if required.

Contract: Permanent
Hours: Mon-Fri, 15-19 hours per week. Afternoons initially. Flexible schedule.
Salary: Dependent upon experience. Please contact glenfamprac@eircom.net to apply or Tel 01 626 0562 for further information
MIXED GMS/PRIVATE PRACTICE FOR SALE
Medium-sized, private, and GMS single-handed practice in the Midwest looking for a qualified GP to join in a shared/flexi contract, with eventual takeover of the practice.

If interested, please contact 087 285 7455 or email: nnajiofor@gmail.com
Principal retiring shortly
PRACTICE/CLINIC NURSE, DUBLIN 14
We are a unique general practice using a blend of conventional medicine and cutting-edge functional medicine to help our patients return to vibrant health. Mostly we see patients with a variety of conditions including CFS, IBS, fibromyalgia, severe hormonal imbalances, and life-wrecking autoimmune diseases. We also use innovative therapies including IV nutrition and ozone therapy. Our team is friendly, close knit, and enthusiastic. There is job satisfaction in buckets.
The role will include patient care, phlebotomy, and administration of intravenous therapies. The hours are 8-12 pw, mostly afternoons, plus occasionally an extra 8-10 hours of morning work for colleague cover.
Comprehensive training will be provided. You will be someone who enjoys variety at work, has a sense of humour, intellectual curiosity, and a kind heart.
Please see www.drummartinclinic.ie or to speak with our nurse, Kathryn, phone: 01 296 5993.
Please send your CV with covering letter to vacancies@ drummartinclinic.ie with NURSE in the subject line
Classifieds & Recruitment THE MEDICAL INDEPENDENT | 31 MAY 2021 39 To advertise, email Louis at louis@mindo.ie ARE YOU LOOKING FOR GP COVER? LOCUM, LONG-TERM OR PARTNERSHIP GET IN TOUCH WITH MATCHMEDICS TODAY TO FIND YOUR NEXT HIRE! CALL GRAHAM ON 087 758 4592 Email: graham.cosgrave@matchmedics.com
If you have anything you would like to share, please email: info@mindo.ie


A round-up of news and oddities from left field by
Dr Doug Witherspoon
From the bedside to the court bench –when the law becomes an ass
It appears the Covid-19 pandemic has led to a significant drop in the amount of medical negligence cases in the past year. While there are cases which have merit, there are others which are opportunistic and frivolous. Either way, Covid has taken the wind from the sails of the courts services, which are just starting to lumber back into motion on a widespread basis now.
Some negligence cases should of course never even make it to court, but such is the world we live in, and a dip into the archives shows how shockingly absurd some of these cases have been, particularly in the US.
Notwithstanding the severe stress and potential reputational damage that physicians suffer from such cases, some are so bizarre that they deserve a mention.
One such case involved hand surgeon Dr Ted Grenga of
called to attempt to reattach a dismembered hand. Here's the sketchy case study: The patient, Mr Thomas Passmore, was a construction worker who unfortunately had a long history of psychiatric illness. Mr Passmore perceived that he had "666, the sign of the devil" manifest on his right hand, which he perceived to be a sign of demonic possession and therefore he used a power saw to slice off his right hand. Workers at the site acted quickly to put the hand on ice and both Mr Passmore and the hand were transported by helicopter to the hospital.
The patient gave his consent for the reattachment procedure and seemed coherent, but Dr Granga asked for the opinion of a psychiatrist, who deemed that the patient had the mental and legal capacity to provide his consent for the procedure. All good so far.
surgery and being wheeled into theatre, he withdrew consent and according to defence attorney John R Fitzpatrick, "he said that if Dr Grenga reattached his hand, he'd cut it off again." The psychiatrist was again called upon and decided that the sedatives had not significantly affected the patient's ability to provide or withdraw consent. Needless to say, time was now of the essence: "The operation needed to be performed as soon as possible for any chance of success and Dr Grenga knew that self-mutilators have a high propensity to do it again," said Mr Fitzpatrick.
The hospital risk manager and a local judge were inserted into the situation and asked for advice and guidance and as Mr Fitzpatrick explained at the time, "the judge said that as long as a psychiatrist certified that Passmore was competent, Dr Grenga couldn't perform the operation against the man's will. If he did, he could be charged with criminal assault and sued civilly as well." Blimey.
Dr Grenga counselled the patient that the longer the delay, the less chance the hand could be reattached, but he again refused consent. The surgeon therefore had no choice but to close the wound and that was the end of the matter from a medical point of view.
However, not too long afterwards, Dr Grenga received notification from a lawyer that Mr Passmore would be suing the surgeon and hospital to the tune of $3 million on the basis that the surgeon should have known that he was mentally unstable and therefore did not have the capacity to give or withdraw consent and Dr Grenga should have reattached the hand, regardless of what his patient said.
You might think the case should have not gone to trial at all, but as Mr Fitzpatrick explained, "you may wonder how a case this frivolous was allowed to proceed, but the plaintiff's attorney had expert witnesses lined-up, saying that the surgeon and hospital should have operated. As long as an expert was willing to testify, the judge felt he had to let the case go on."
The case lasted nine days and it was reported to be "surreal", according to a report in Medscape , with the plaintiff raising a hook at the end of his right arm when being sworn-in.

Dr Grenga's defence cost his insurer north of $70,000 and Mr Fitzpatrick noted that "Passmore also sued the psychiatry group at the hospital, which settled before trial for an amount believed to be in the mid-six figures". In between the reels and the jigs, a jury took only 30 minutes to exonerate Dr Grenga and one or two even complimented him on being a good doctor.
The last word goes to an exasperated Mr Fitzpatrick: "I disagree that frivolous suits are a thing of the past. It's easy to find an expert witness to advance bogus theories. Plaintiffs' attorneys know that most cases settle and they figured Dr Grenga would settle to avoid the nuisance and risk of the lawsuit."
The Dorsal View THE MEDICAL INDEPENDENT | 31 MAY 2021 40













































































































































































































