

MORE THAN 90% YEAR OLDS IN IRELAND GET ENOUGH VITAMIN D
MORE THAN OF 1-3 YEAR OLDS DO NOT GET ENOUGH
MILK
APTAMIL TODDLER MILK
APTAMIL TODDLER MILK


for Toddlers 2020 drinks may contribute to the Iron & Omega 3 in toddlers2 supplementation of 5μg is Halloween to St. Patrick’s Day*2 (300ml) of Aptamil Toddler with 100% of the RDA3 for RDA3 for Iron possible.
FSAI Dietary Guidelines for Toddlers 2020
FSAI Dietary Guidelines for Toddlers
• Fortified foods and drinks may contribute to the intakes of Vitamin D, Iron & Omega 3 in toddlers2
• Fortified foods and drinks may contribute intakes of Vitamin D, Iron & Omega
• Daily Vitamin D supplementation of 5μg is recommended from Halloween to St. Patrick’s Day*2
• Daily Vitamin D supplementation recommended from Halloween to
Just 2 beakers a day (300ml) of Aptamil Toddler milk provides toddlers with 100% of the RDA3 for Vitamin D and 53% of the RDA3 for Iron
Just 2 beakers a day (300ml) of Aptamil milk provides toddlers with 100% of Vitamin D and 53% of the RDA3 for


*Along with fortified foods and drinks where possible. Available in 800g powder, 200ml & 1 litre liquid.
Alternatively, call our dedicated visit nutricia.ie
balanced
Scan for more information. Alternatively, call our dedicated freephone on 1800 22 12 34 or visit nutricia.ie


Scan for more information. Alternatively, call freephone on 1800 22 12 34 or visit nutricia.ie
This information is for healthcare professional use only. Aptamil Toddler Milk should be used as part of a varied and balanced diet
is
in Ireland. Available at: https://www.fsai.ie/Dietary_Recommendations_1-5_Year_Olds/ 3. Food Safety Authority of Ireland (FSAI), Recommended Dietary Allowances for Ireland 1999. Available at: https://www.lenus.ie/handle/10147/44808
*Along with fortified foods and drinks where possible. Available in 800g powder, 200ml & 1 litre liquid. January 2022
FOCUS ON MEN’S HEALTH
Welcome to the July/ August edition of Nursing in General Practice. The focus of this edition is on men’s health.
International Men's Health Week (MHW) ran from Monday 13 June until Father’s Day on Sunday 19 June. MHW is celebrated in many European countries, as well as in the US, Australia, New Zealand, Canada, and a number of other places worldwide. The overall aims of the week are to:
1. Heighten awareness of preventable health problems for males of all ages.
2. Support men and boys to engage in healthier lifestyle choices/activities.
3. Encourage the early detection and treatment of health difficulties in males. During 2022, the theme for Ireland was 'MISSION: isPOSSIBLE' and the call to men (and those who support the health of men) was: 'The action starts with you.'
The Men’s Health Forum in Ireland (MHFI), the body that co-ordinates MHW, is a diverse network of individuals and organisations, men and women, whose aim is to:
Identify the key concerns relating to male health;
Increase understanding of these issues;
Address the impact of this inequality.
MHFI works on an all-Ireland basis to enhance the health and well-being of men and boys through research, awareness raising, training, networking, and practical demonstration projects.
During MHW 2022, everyone was asked to make new choices and to realise that where there’s a will, there’s
a way. MHW provides everyone (health professionals, service providers, sporting bodies, community groups, employers, policy makers, the media, churches, individuals) an opportunity to encourage men and boys to take better care of their health and to seek help or treatment at an early stage.
The MHFI coordinates activity on the island of Ireland and cooperates with other men's health fora across Europe, and further afield, to mark this week.
The celebration of MHW on the island of Ireland in 2022 was funded by the HSE Health and Wellbeing, Healthy Ireland within the Department of Health, and the Public Health Agency in Northern Ireland.
Males constitute almost 50 per cent of the population on the island of Ireland and, therefore, deserve to have a gender lens focused upon their specific
health needs. Research clearly shows that men experience a disproportionate burden of ill-health and die too young:
Men die younger than women.
Males have higher death rates than females for almost all of the leading causes of death and at all ages, eg, cardiovascular disease, cancer, etc.
Men’s poorer lifestyles are responsible for a high proportion of chronic diseases.
Late presentation to health services can lead to many problems becoming untreatable.
In this issue, Theresa Lowry-Lehnen discusses two topics of particular importance to men – testicular cancer and erectile dysfunction, while there is also an update on a new Irish Cancer Society trial for male cancer patients. Other articles featured include melanoma prevention and early detection, bladder pain syndrome/ interstitial cystitis, and part two of a CPD module on diabetes.

I hope you all have an enjoyable summer and have some time off to rest and recharge.
NiGP is now a fully independent publication and is no longer the official journal of the IGPNEA. If you are interested in writing an article for NiGP, please email priscilla@mindo.ie.

C ONT ENTS
04
11
NEWS
Irish healthcare news
BLADDER PAIN SYNDROME
Urogynaecology specialists Prof Barry O’Reilly and Dr Yair Dayka discuss the presentation and supportive treatment approaches for bladder pain syndrome/interstitial cystitis
14
CPD: TYPE 2 DIABETES
In part two of a two-part CPD module, Theresa Lowry-Lehnen outlines the presentation, diagnosis, and treatment of type 2 diabetes in the community
21
RHEUMATOLOGY
Niamh Quinlan provides a round-up of stories from the Irish Society for Rheumatology Spring Meeting
24
26
WOMEN’S HEALTH
An update on new resources for the menopause and the Government’s latest women’s health initiatives
MEN’S HEALTH
EDITOR
Priscilla Lynch priscilla@mindo.ie
CONSULTING EDITOR
Ruth Morrow
SUB-EDITOR
Emer Keogh emer@greenx.ie
CREATIVE DIRECTOR
Laura Kenny laura@greenx.ie
ADVERTISEMENTS
Graham Cooke graham@greenx.ie
ADMINISTRATION
Daiva Maciunaite daiva@greenx.ie
Please email editorial enquiries to Priscilla Lynch priscilla@mindo.ie
Nursing in General Practice is produced by GreenCross Publishing Ltd (est. 2007).
© Copyright GreenCross Publishing Ltd. 2022
Please email publishing enquiries to Publisher and Director, Graham Cooke graham@greenx.ie
Theresa Lowry-Lehnen gives a comprehensive overview of the presentation, diagnosis, and treatment of erectile dysfunction 31
34
MELANOMA
Dr Una Kennedy, GP Advisor to the National Cancer Control Programme, and colleagues, discuss prevention and early diagnosis strategies for melanoma skin cancer
MEN’S HEALTH
Theresa Lowry-Lehnen outlines the presentation, diagnosis, and latest treatment approaches to testicular cancer
39
FOOD AND WINE
Tom Doorley discusses the health impact of gut flora
40 CROSSWORD
Test your word knowledge

The contents of Nursing in General Practice are protected by copyright. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means – electronic, mechanical or photocopy recording or otherwise –whole or in part, in any form whatsoever for advertising or promotional purposes without the prior written permission of the editor or publishers.
DISCLAIMER
The views expressed in Nursing in General Practice are not necessarily those of the publishers, editor or editorial advisory board. While the publishers, editor and editorial advisory board have taken every care with regard to accuracy of editorial and advertisement contributions, they cannot be held responsible for any errors or omissions contained.
Artrial Fibrillation
On completion of this module, the reader should have an enhanced understanding of atrial fibrillation, including different types of arrhythmia, diagnostic methods, and the drugs and procedures that can be used to treat the condition.
NEW IRISH CANCER SOCIETY MEN'S TRIAL
Anew €300,000 Irish Cancer Society research trial aimed at helping make crucial improvements to quality-oflife for men facing devastating cancer diagnoses has been launched.
When a man has cancer, attention immediately turns to providing lifesaving treatment, but all too often issues arising from their treatment can be hugely detrimental for their actual quality-of-life – issues like weight gain, muscle loss, continence problems, and erectile dysfunction.
For the first time in Ireland, this project will see men routinely linked into a range of supports in a hospital setting that can help them live their lives to the fullest throughout their diagnosis.
Known as the Irish Cancer Society Liam Mc Trial, short for ‘Linking in with advice and supports for men impacted by metastatic cancer’, the name is also a nod to Cork man Liam McCarthy whose name is borne by the All-Ireland hurling trophy.
A 12-week programme developed as part of the Liam Mc Trial will see initial groups of selected participants with an advanced cancer diagnosis receive dedicated help from a range of hospitalbased specialists, including everything from physiotherapy and psychology to dietitians and nursing.
The programme is specifically tailored to the needs of common male cancers such as prostate, testicular, and bladder cancer, with input from patients key to its design. Its various components will cater for the psychological, emotional, and physical needs of men affected.
Based out of the state-of-the-art cardio rehab gym at Cork University Hospital (CUH), it will be supported
by the University College Cork (UCC) Cancer Trials Group and overseen by a team of researchers from UCC and CUH under the direction of Consultant Medical Oncologist Dr Richard Bambury, and Lecturer Practitioner in Nursing Dr Brendan Noonan.
Participating men will receive an assessment at the beginning of the 12week programme, at the halfway point once they have begun receiving supports, and again at the end of the 12 weeks.
They will be followed up with after six months to measure overall improvements in quality-of-life, with the aim of creating a new standard of care for such patients that can be replicated nationwide.
Commenting on the launch of the Liam Mc Trial, Dr Bambury said: “We are delighted to open this hugely important trial with the support of the Irish Cancer Society that will investigate the role of a targeted 12-week personalised programme for men impacted by metastatic cancer, focusing on a predominantly underserved and under-represented patient group in this setting.
“Building on the success of the LYSA trial in Cork, investigating women's survivorship strategies, this study is aimed at men who are survivors of cancer. These men will receive dedicated interventions and supports from a multidisciplinary team, including physiotherapy, dietitian, nursing, psychological, emotional, and medical inputs, with the ultimate aim of improving their quality-of-life.”
Irish Cancer Society Research Manager Dr Claire Kilty said: “When men are diagnosed with cancer there is naturally an immediate focus on making sure they survive, but the quality-of-
life from that point on is also incredibly important for men. We hope this new model will support them to live full and fulfilling lives that are so integral to personal wellbeing."
The Liam Mc Trial was launched to coincide with Men’s Health Week, which took place from 13-19 June.
The initiative has grown in popularity over the last number of years, with many organisations and individuals lending their support in order to highlight the different health and wellbeing topics discussed over the week. HSE Health and Wellbeing as a key funder of the Men’s Health Forum in Ireland, the body that co-ordinates Men’s Health Week, is proud to be associated with Men’s Health Week, which aims to raise awareness of preventable health problems, support men and boys to live healthier lives, and encourage them to seek help or treatment at an early stage. In recent years, a broad range of research has highlighted the challenges which face males in Ireland and further afield.
Many of the key statistics, highlighted in the ‘Men’s health in numbers’ publications, show that:
Men continue to die, on average, younger than women do.
Poor lifestyles (including smoking, drinking, diet, and lack of exercise) are responsible for a large proportion of chronic diseases.
Males have higher death rates than women for almost all of the leading causes of death and at all ages.
Men’s mental health needs are often under the radar and remain unmet.
Late presentation to health services can lead to a number of problems becoming untreatable.
Increasing role of GPNs highlighted at WONCA

Advanced nurse practitioner in general practice and PhD candidate Orla Loftus Moran presented the details of her (and her colleagues) scoping review on the role of general practice nursing in Irish general practice at the WONCA World Rural Health Conference 2022, which took place from 17-20 June at the University of Limerick.
The review is the first stage of her PhD research, which ultimately aims to develop a suite of nursing quality care process metrics specific to general practice nursing. Irish general practice nursing roles have developed and grown
exponentially in response to changing policy, clinical and workforce demands she explained, adding that as nursing care in general practice advances at pace, comprehensive evaluation of the GPN role has not been undertaken to date.
Irish GPNs lead and deliver a broad range of the healthcare services, including primary infant and adult immunisation programmes, cervical screening, health promotion and education interventions, disease surveillance, acute and minor illness triage and management, wound care, and a complex range of generalist nursing services. Furthermore, GPNs adapted to challenges in delivery of
services resulting from the Covid-19 pandemic, and were integral to continuation of services, particularly the delivery of the Covid-19 vaccination programme in general practice.
International research demonstrates that mobilisation of the GPN workforce and advanced GPN roles can strengthen general practice, whilst providing benefits and safe care for patients, additionally this research suggests that GPN roles may be underutilised, Ms Loftus Moran, who is one of two recipients of the Judith Chavasse 2021 Scholarship awarded by the School of Nursing, Midwifery, and Health Systems, UCD, outlined.
HCPs need to improve at tackling misinformation – WHO Executive Director
Healthcare professionals should improve at early community engagement to provide accurate information about major health measures, according to the Executive Director of the World Health Organisation's (WHO’s) Health Emergencies Programme, Dr Mike Ryan.
Dr Ryan spoke at the WONCA World Rural Health Conference 2022, at the University of Limerick Concert Hall via live video chat. Answering a question from the audience about misinformation, Dr Ryan said healthcare professionals need to be “open and transparent” with the public and to “address concerns where they are” to combat misinformation and disinformation.
“I think that's something that we need to get better at within the medical profession and within the health profession in general; engaging early with communities and explaining why things are being done,”
he said. “And getting much better at being the first ones to communicate, rather than reacting all the time to the negative spin that's put on things.”
When large-scale health and social measures, such as shutting down schools and public transport, are implemented, there is a major impact on societies. There needs to be “open and robust discussion with communities” around these, or misinformation and disinformation can move in with false explanations, he maintained.
Dr Ryan said: “[That] is a whole other part that I'm still trying to come to terms with myself… the active manipulation of health information.”
The “anti-vaccination” movement, he believes, particularly in Ireland, is in a minority. “Most of the anti-vaccination that I've come across is a misattribution of antivaccination to someone who has genuine questions about vaccination. And that is hesitancy driven by a concern or a lack of information.” As a result, information needs to
be clearly explained, he said.
“The number of individuals who are actively producing the misinformation and disinformation; they're the ones we need to get after. Because they're the ones that are poisoning people's minds… I don't mean physically get after them, I mean we've got to counter what they're saying, we've got to put out better [information], we've just got to get much better at doing what we do.”
He said that the pandemic had exposed a lot of the frailties in the system; “a lot of the access issues in the system, a lot of the justice issues in our health and social systems that many of you have been speaking about for years,” Dr Ryan told the WONCA audience. “But they have been pretty brutally exposed in the Covid response.”
The pandemic exposed the need to improve on the 'Five Cs', he told NiGP: Community protection; Clinical care; Collaborative surveillance; access to Counter measures; and Coordination. However, he said no improvements have been made on these.
‘NECESSITY IS THE MOTHER OF INVENTION’
Two
nurses win top award for their innovative 'Wee Catch It' idea
Two nurses at the Department of Public Health Mid-West have received a top award in recognition of their innovative idea to collect urine samples in a cleaner way in order to improve diagnosis rates of infections, with the aid of a special app.
Public Health Mid-West’s Assistant Director of Nursing (ADON) Bernadette Higgins, and Clinical Nurse Manager 2 (CNM2) in Health Protection Anne
Murray won the National Spark Ignite award and €3,000 seed funding for their ‘Wee Catch It’ innovation idea at the annual Spark Innovation Programme conference in Dublin last month.

The Spark Ignite award seeks innovative ideas from the 115,000+ HSE employees aiming to improve patient and healthcare outcomes. Spark Ignite is open to all disciplines and departments within the HSE, enabling staff to develop their ideas through validation of clinical need and to determine the market for their proposed solution, product, or service. Successful applicants will also receive guidance on how to bring their ideas towards reality.
Collecting a clean, accurate midstream urine in babies, children, and adults with incontinence in an efficient and hygienic way is challenging; this can lead to contamination of samples and often results in unnecessary antibiotic prescribing and costly unplanned hospital admissions. Necessity being the mother of invention, Bernadette came up with a solution to improve the ‘clean catch’ urine samples and targeting antimicrobial stewardship with ‘Wee Catch It’.
Bernadette and Anne had previously
won the Regional Spark Ignite award and €3,000 seed funding in early June.
‘Wee Catch It’ is currently in prototype development phase and the public health nurses will now work towards developing the unique product and software for national and international use.
Bernadette Higgins, ADON, who hails from Longford and lives in Clare, said that she is delighted with the recognition and looks forward to taking the idea to the next stage.
“Being shortlisted and winning the Spark Ignite Award is an honour considering the exceptional standards of all the other entrants. Winning this prestigious award in such a high calibre competition has given the validation to believe in the innovation MedTech device ‘Wee Catch It’ and drive it forward. We are an example of how passionate,
creative nurses are in an ideal position to invent and develop ideas as we spend more time with patients. Nurses have a wealth of knowledge of how products work and can make patients’ and healthcare workers’ lives better. The ability for a nurse to innovate is defined by a desire to improve some of the most vital solutions in healthcare,” said Ms Higgins.
Anne Murray, CNM2, from Cork and living in Kilcornan, County Limerick, said: “Nurses have always been unintentional innovators. Improvising, moulding, and tweaking what was available to us. They bring true meaning to the phrase ‘necessity is the mother of invention’ in their efforts to optimise the care they give. In recent years, healthcare recognises the enormous value of fostering creativity and innovation in healthcare workers. We are so incredibly honoured and humbled to have been chosen from such an incredible group of passionate SPARK finalists, all of whom had amazing innovations. Without the HSE’s SPARK programme, our innovation would still be just an idea.
“As a parent, I know the distress of trying to get a urine sample from a baby. As nurses, our patients are the centre of all we do. We want to be able to deliver the best patient experience we can in an effective, efficient, simple, comfortable, safe, and cost-effective way. ‘Wee Catch It’ does all this. The idea is that it will improve the process for the healthcare worker, provides education, promotes appropriate and timely treatment, and reduces the risk of harm to the patient and the wider population.
For or to avail freephone


IMPORTANT NOTICE: Breastfeeding is best. Aptamil Pepti Syneo is a food for special medical purposes for the dietary management of Cow’s Milk Allergy. It should only be used under medical supervision, after full consideration of the feeding options available including breastfeeding. Suitable for use as the sole source of nutrition for infants under one year of age. Refer to label for details.

IMPORTANT under medical of age. Refer
* Short chain galacto-oligosaccharides / long chain fructo-oligosaccharides; ^Single arm UK study in infants with non-IgE mediated CMA, baseline non-synbiotic formulas vs Aptamil Pepti Syneo, 4 week intervention; † Subgroup of n=48 infants with IgE associated AD, difference in SCORAD score Aptamil Pepti Syneo vs EHF without synbiotics, -4.6, 95%CI, p=0.04. ‡ Products can be provided to patients upon request of a healthcare professional. They are intended for the purpose of professional evaluation only. 1.


* Short chain Pepti Syneo, ‡ Products can
2. Giampietro 5. Browne et BRI, October Nutricia Ireland,
‘NO WAITING LISTS!’ –
NATIONAL MENTAL HEALTH CHARITY URGES PUBLIC TO USE ITS SERVICES
Turn2Me, a national mental health charity, has urged the public to use its free mental health services, following the recent publication of an Economic and Social Research Institute (ESRI) report, which classified 55 per cent of 22-year-old women and 40 per cent of 22-year-old men as ‘depressed’.
Turn2Me offers adult counselling and peer support services to adults and young people aged 12 plus. It also offers group support services for teenagers, young people, and adults. The charity emphasised that two of its free services; its support groups and its ‘Thought Catcher’ service, have no waiting lists and can be used by anyone in Ireland or the UK.
Turn2Me stated that the results of the ESRI report are shocking and are reflective of what mental health professionals witnessed during the Covid-19 pandemic. Demand for Turn2Me’s services significantly increased during the Covid-19 lockdown – at the peak of the pandemic, demand for its services spiked by 386 per cent.
Turn2Me is urging anyone who is experiencing anxiety, depression, grief or relationship issues to sign up for their free support groups, which are facilitated by mental health professionals and run six evenings a week.
The ‘Thought Catcher’ service runs every day from 2pm until 8pm. It is a safe online platform where people can post about how they’re feeling, and other users can respond with positive, uplifting messages. Users can engage with both the 'Thought Catcher' and the support groups without the barrier of a waiting list. These services are funded
by the HSE National Office for Suicide Prevention (NOSP).
“The ESRI report is shocking,” Fiona O’Malley, CEO of Turn2Me, said. “It clearly outlines how the pandemic negatively affected so many people, particularly young people. I would encourage anyone who is feeling depressed to engage with our free mental health services, particularly our support
groups and our 'Thought Catcher' –both these services are free, operated by mental health professionals and don’t have long waiting lists attached!”
People can sign up to Turn2Me’s free mental health services by going to www.Turn2Me.ie, setting up a free account, and signing up for the support groups or engaging with the ‘Thought Catcher’.
NMBI announces introduction of Humanitarian Practice Permit
Nurses and midwives from outside Ireland who wish to practise on a temporary basis in Ireland can now apply for a permit to do so, the Nursing and Midwifery Board of Ireland (NMBI) has announced.
The move follows the signing of a commencement order by Minister for Health Stephen Donnelly, which brings into operation Sections 39 and 41 of the Nurses and Midwives Act 2011.
The legal change will allow practising nurses and midwives visiting Ireland for work purposes to apply for a Humanitarian Practice Permit in the following circumstances:
A. Patient transfer (emergency and acute clinical care).
B. Training that requires the nurse/ midwife to be in a clinical area.
C. Accompanying a client/person for a sporting event.
D. Patient support for short-term respite care.
A person qualified to practise in a place outside the State and in the State for a humanitarian purpose may be issued with a permit by the NMBI allowing them to practise for a period not longer than 30 days. This permit provision does not apply to nurses and midwives who are eligible to have their qualifications recognised under
RECRUITMENT
PRACTICE NURSE WANTED
GPs at Tallaght Cross are seeking an enthusiastic, empathetic, and professional nurse to join our team. We are fully computerised with experienced administration staff, caring for a thriving, pleasant and friendly local population. Regular duties will include:
Phlebotomy Primary childhood and adult immunisations CervicalCheck
ECG/ABPM/Holter Chronic disease management programme (knowledge of diabetes)
Wound care Suture removal Aural care
Manage clinical stock Cold chain and administration of State schemes
Qualifications and skills:
RGN Registered with NMBI Practice nurse experience ideal Minimum of 5 years post qualification experience.
Contract length: 12 months
Hours of work – negotiable. Competitive salary. Contact Ciara.mccarthy@tallaghtcrossdoctors.ie to apply or telephone 087 205 6885 for further information
PART-TIME PRACTICE NURSE REQUIRED
2/3 days per week or 2 to 4 mornings. Immediate start. Days flexible. Friendly environment and good hourly rates for right candidate.
The role includes:
Chronic disease management and organisation Vaccinations: Infant/child, adult, and travel Phlebotomy,
Cervical smear taking Dressings/ wound care. ECG/blood pressure assessments/Holter monitor
Ear syringing Equipment maintenance and clinical stock management
Candidate requirements:
Must be a registered general nurse with the Nursing and Midwifery Board of Ireland. Send CV to sladecastle.gp@gmail.com
the European Union (Recognition of Professional Qualifications) Regulations 2017 (S I No 8 of 2017) and who, accordingly, are eligible to apply to provide services on a temporary and occasional basis.
CEO of NMBI, Sheila McClelland, said: “We are pleased to announce that nurses and midwives who are registered within their home state may now apply for a Humanitarian Practice Permit.
“This permit is meant for nurses and midwives involved in care provision for an isolated practice episode. Should a nurse or midwife wish to practice on a regular basis they will need to apply to NMBI to join the Irish register.”
REGISTERED SMEAR TAKER REQUIRED
Registered smear taker required for 1-2 sessions per week for a busy women’s health clinic, Dublin 15. Flexible hours/days excellent rate of pay. Email your CV to info@femplus.ie
If you would like to place a recruitment advert in the next edition, please contact Louis@mindo.ie
HSE LAUNCHES PRACTICAL RESOURCES FOR PARENTS AND THOSE WORKING WITH YOUNG CHILDREN
The HSE, in partnership with Tusla, aims to promote and support the understanding of infant mental health among parents and those working with young children across Ireland.
A series of 10 practical videos with HSE expert advice are now available and can be accessed at https://rb.gy/sb8xem. The videos were launched to coincide with Infant Mental Health Awareness Week 2022, which took place from 13-19 June.
Infant mental health refers to how well a child develops socially and emotionally from conception to age three years, in the context of their family, their community and their culture. This includes their capacity to express and regulate their emotions, begin to form relationships, and explore their environment.
Dr Audrey Lonergan, Principal Psychologist, and Infant Mental Health Specialist HSE, explains the importance of prioritising infant’s mental health: “Early parent-infant interactions during the first weeks, months, and early years of a child’s life are very important in building the architecture of the brain and lay a strong foundation for healthy social and emotional wellbeing across the lifespan. By paying attention to infant mental health, we can help establish a positive trajectory for the child’s development.”
The suite of videos feature HSE experts in child psychology highlighting advice when it comes to supporting and promoting infant mental health, as well as parents sharing and reflecting on their own experiences.
Dr Lonergan continues: “These handson, practical videos offer advice for new parents on bonding with your baby, as well as connecting and communicating with them, how to help your baby regulate their emotions, how to respond to your
baby’s cues, including crying, and how to settle your baby for sleep. For parents of toddlers and young children, parents can learn about how to follow your child’s lead in play, the importance of storytelling and tips around settling your child into childcare. These videos support and encourage parents or caregivers by emphasising how everyday moments with their baby can support their social, emotional and brain development.
Catherine Maguire, Senior Clinical Psychologist and Infant Mental Health Specialist with Childhood Matters, said: “These videos are developed to also highlight the importance of relationships in an infant and young child’s life, beginning within the parents and family, extended family and early years providers. All development happens within the context of a relationship; the nature, quality, and consistency of these relationships lay the foundation for practically all aspects of development that follow.”
It is particularly important that parents don’t create unrealistic expectations for themselves and their babies; good enough parenting is what matters, she added. Being a new parent can be difficult at times and some parents may need extra guidance. GPs and general practice nurses (GPNs) are often the first port of call for new parents seeking support and reassurance.
Expert tips for parents of babies
Everyday interactions strengthen and develop your bond with your baby- just looking at each other, reading stories, singing, and smiling – help to build a connection and will let your baby know that you are there for them.
Every time you interact with your
baby, neural-connections in the brain are being laid down. These are necessary to build the foundations of their social, emotional, and cognitive development, including language development.
It takes time to get to know your baby. Through everyday interactions you will learn what your baby is trying to communicate, you will learn to read their cues.
Responding to your baby at these times is not spoiling your baby – rather it will help to build their sense of trust and security in their relationship with you.
Expert tips for parents of toddlers and young children
This can be a challenging stage, as toddlers begin to assert their independence. They struggle to manage very strong feelings. Your toddler still needs lots of help to manage these strong feelings. Responding to them in a calm way and naming their feelings can really help your toddler to cope and to feel understood.
Toddlers learn by exploring their environment and playing. It is helpful to try to follow your child’s lead in play. This means stepping back and allowing them to use their imagination and ideas. Wait to see what they are interested in and listen, respond, and comment on what your child is doing. This helps to promote self-confidence, self-esteem, and good communication skills in your child.
Transitioning your child to childcare can be challenging. Preparation is key. Practice routines around goodbyes and reunions to create predictability and help them cope in this time of change. You can also read stories about going to childcare or visit the childcare centre beforehand.
Lots more expert advice for every step of pregnancy, baby and toddler health can also be found at www.mychild.ie.
BLADDER PAIN SYNDROME/ INTERSTITIAL CYSTITIS
INTERSTITIAL CYSTITIS IS A PAINFUL, DEBILITATIVE CONDITION AND WHILE INCURABLE, TREATMENTS ARE AVAILABLE TO IMPROVE QUALITY-OF-LIFE
Bladder pain syndrome, also known as interstitial cystitis, involves bladder pain or discomfort that can profoundly impact quality-of-life.
Bladder pain syndrome is challenging to diagnose, prevent, and treat because its basic science and pathophysiology are poorly understood. The prevalence of bladder pain syndrome varies widely and is estimated to be from 1-to-20 per cent. It is estimated that 90 per cent of people with interstitial cystitis are women.
The symptoms of bladder pain syndrome are discomfort with a frequent and often urgent need to pass urine. The findings of interstitial cystitis are suprapubic or retropubic pain, pressure, or discomfort related to the bladder. The pain usually increases with intensity during bladder filling and may persist or disappear after voiding.
To date, there is no curative treatment and the primary goal of management is to provide symptom relief to achieve an adequate quality-of-life. There are many therapeutic approaches for bladder pain syndrome, none of which have been proven helpful for all patients.
Women with bladder pain syndrome also have other associated urinary symptoms and a higher prevalence of specific systemic diseases (eg, inflammatory bowel disease).
People with interstitial cystitis may have many of the following symptoms:
X An urgent need to urinate, both in the daytime and at night.
X A frequent need to urinate as many as 20 times a day or more.
X Pressure, pain, and tenderness around the bladder, pelvis, and perineum may increase as the bladder fills and relieves as the bladder empties.
X The sensation that they can’t hold as much urine as before.
X Pain during sexual intercourse.
Risk factors
X Sex: Women are diagnosed with interstitial cystitis more often than men.
X Age: Most people with interstitial cystitis are diagnosed during their 30s or older.
X Chronic pain disorder: Interstitial cystitis may be associated with other chronic pain disorders such as irritable bowel syndrome or fibromyalgia.
X Stimulatory foods/drinks: Spicy foods and caffeine.
Pathophysiology
The pathophysiology of interstitial cystitis is complex and includes a number of different possible aetiologies. The main points are epithelial dysfunction, immune system activation (mast cells), neurogenic inflammation, autoimmunity, and occult infection. One of the most common findings is the thinning of the bladder epithelium, suggesting injury and damage to the blood supply.
Other changes seen include increased histamine (produced due to the

inflammation process) and increased nerve cells in the bladder wall. An autoimmune response (when antibodies are made that act against a part of the body, such as in rheumatoid arthritis) may also be the cause in some people.
The urothelium, which is the inner surface of the urinary bladder, acts as a barrier between urine and the underlying bladder. The bladder mucus is a critical component of this function and is composed of glycosaminoglycans (GAGs), which are highly hydrophilic and trap water at the cell’s outer layer. The primary pathology of the disease is a disruption of the urothelial barrier, the penetration of the urinary solutes (potassium), causing injury to the nerves and muscles.
Evaluation
Clinical symptoms: This condition is suspected in patients who have pain in the urinary bladder for a few weeks. Specific findings include frequent urination to avoid discomfort, with bladder distension and pelvic tenderness on examination.
Physical examination: Tenderness or tightness of the pelvic floor muscles, which can be identified by palpation of the levator muscles on pelvic examination, is consistent with the diagnosis.
Findings on examination that require workup for other diagnoses include significant pelvic prolapse, urethral diverticulum, uterine/cervical mass, and eroded/exposed vaginal mesh.
Additional testing
Urine tests: Urinalysis and post void residual (PVR) urine volume.
Urinalysis with microscopy is performed to rule out infection and check for haematuria. A urine culture should be performed if urinalysis results suggest urinary tract infection. A PVR of urine can be measured by ultrasound
Cystoscopy: Cystoscopy is not a mandatory tool to make the diagnosis of interstitial cystitis. However, it may be performed to exclude other aetiologies either on initial presentation or in patients who do not respond to treatment with oral medications, and can help in the treatment. Over 50 per cent of patients may experience some symptom relief, but this will rarely last longer than six months. Cystoscopy can be used for cystodistension of the bladder and fulguration of Hunner’s ulcers/lesions.

There are typical findings of lesions related to interstitial cystitis during cystoscopy: Hunner’s lesions (reddened lesions on the bladder mucosa with attached fibrin deposits; typically bleed after

hydrodistension) in 5-to-10 per cent of patients, and are highly specific. To identify Hunner’s lesions, cystoscopy is performed with direct visualisation before and after hydrodistension, and biopsies are taken of suspected lesions (see Image 1).


Glomerulations (punctuate petechial haemorrhage), which appear after hydrodistension (see Image 2).
Both can be found in a patient with interstitial cystitis, but not all patients with interstitial cystitis will have them (not reliable criteria).
Histology
Increased numbers of mast cells on histologic examination of bladder biopsy specimens.
Biomarkers of interstitial cystitis
X GB-51.
X HB-EGF.
X APF (anti proliferative factor) is emerging as the best candidate for a biomarker for interstitial cystitis, but further studies and trials are needed. Excluding other aetiologies is essential.
Diagnosis
The American Urological Association (AUA) guidelines use the following definition of interstitial cystitis/painful bladder syndrome: “An unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks duration, in the absence of infection or other identifiable causes.”
The clinical diagnosis requires a careful history, physical examination, and laboratory testing to document the disorder’s basic symptoms and exclude infections or other disorders.
Differential diagnosis
X Bladder or urethral cancer.
X Infections.
X Benign pelvic abnormalities.
X Intravesical pathology.
X Urethral diverticulum.
X Neurologic conditions.
X Chronic pelvic pain syndromes.
Treatment
There is no curative treatment for interstitial cystitis and the main goal is to manage symptomatic relief in order to achieve an adequate quality-of-life. Effective pain management is an essential component and may require a multidisciplinary, multimodal approach.
There are many therapeutic approaches, none of which have been proven helpful for all patients. The decision to start pharmacological treatment must be individualised after shared decision-making considering the severity of symptoms, the frequency of flares, patient preference, and the potential adverse effects of continued reliance on analgesic use.
Behavioural/nonpharmacologic treatments
X Patient education.
X Evaluate for comorbid conditions.
X Self-care and lifestyle modification –dietary modification: Avoid acidic foods, coffee, tea, soda, spicy foods, artificial sweetener, and alcohol.
X Pelvic floor myofascial physical therapy.
Pharmacological treatments
X Pain management agents (eg, urinary analgesics, paracetamol, NSAIDs, opioid/ non-opioid medications).
X Amitriptyline, cimetidine, pentosan polysulfate sodium (PPS), hydroxyzine.
X Antihistamines for patients with allergic disorders.
X Neuropathic pain agents.
Intravesical instillations
X Sodium hyaluronate (Cystistat), heparin, or lidocaine may be administered as intravesical treatments.
X Cystoscopy under anaesthesia with short-duration, low-pressure hydrodistension may be considered.
X If Hunner’s lesions are present, fulguration (with electrocautery) and injection of triamcinolone/ Botox should be performed in a second-step procedure.
X Intra-detrusor botulinum toxin may be administered if other treatments have not provided adequate improvement in symptoms and quality-of-life.
Take home message about interstitial cystitis
X Interstitial cystitis is essentially an inflamed or irritated bladder wall.
X The cause of interstitial cystitis is unknown, and antibiotics are not the treatment.
X Symptoms include changes in urination such as frequency and urgency; pressure, pain, and tenderness around the bladder, pelvis, and the area between the anus and vagina or anus and scrotum; and pain during sex.
X There is no best way to diagnose interstitial cystitis.
X A variety of tests may be needed.
X Cystoscopy may be helpful for the diagnosis and treatment
X Treatments are aimed at easing symptoms.
X Lifestyle changes may be advised, and various medications and procedures are available.
TYPE 2 DIABETES MELLITUS ( PART 2)
A COMPREHENSIVE OVERVIEW OF THE PRESENTATION AND MANAGEMENT OF TYPE 2 DIABETES
This article is continued from the May-June issue of Nursing in General Practice.
While healthy eating and exercise are the cornerstones of T2DM management, frequently the addition of medications is needed to help improve blood glucose control. When lifestyle modification fails to achieve the targeted blood glucose levels, the firstline medication prescribed is metformin,18 both for those who are overweight (BMI >25.0kg/m2) and not overweight. Metformin is contraindicated in those with renal impairment and with end-stage cardiac and hepatic failure. Metformin should be stopped in patients with eGFR <30mls/min and at possibly higher values in patients prone to dehydration.10
The Model of Care contains an algorithm with a step-wise treatment approach, with second-line and other agents including DPP-4 inhibitors, sulphonylureas, GLP-1 receptor agonists, pioglitazone, acarbose, meglitinides, sodium glucose co-transporter 2 (SGLT2) inhibitors, and insulin.18
Since 2015, NICE has been advocating a patient-centred approach to glycaemic control and provides best practice advice on setting glycaemic targets and selecting

hypoglycaemic agents for treatment intensification after metformin firstline treatment for T2DM in those with inadequate diabetes control.12
Most guidelines recommend the use of insulin alone or in combination with other glucose-lowering drugs when persons with T2DM are unstable, with sign and symptoms of acute decompensation including dehydration, acute weight loss, acute illness, very high glucose levels, and presence of ketones. Basal insulin should be preferred and it can be temporary. Most insulin algorithms start with 10 units or 0.2 units/kg and titrate once or twice weekly at one to two units each time to achieve a target fasting blood glucose between 3.9 and 7.2mmol/L (70 and 130mg/dL).8
Diabetic Foot Model of Care 2021: T2DM
In 2021 the HSE published its Diabetic Foot Model of Care13 document. The aim of diabetic foot screening and risk classification is to establish the person’s risk of diabetic foot ulceration. All persons with diabetes are assigned a risk category and where appropriate referred for ongoing foot screening, a foot assessment and a clinical care plan. The care plan ensures that all people with diabetes receive annual or more frequent foot screening, foot care education and review, according to their clinical needs and in the most appropriate setting.13
Exception: Those with very complicated T2DM, should have annual foot screening and care provided by the endocrinologist and their team.
There is a full CPD module dedicated to diabetic foot disease available on www.nursecpd.ie.
Diabetic foot screening process: Screening includes:
Based on the findings of this screening the person is categorised as being low-, moderate- or high-risk of future diabetic foot ulceration, or if known to have prior foot ulceration will be categorised as being in remission or categorised as active foot disease, if foot ulceration is present or active Charcot is suspected.13
Complications of T2DM
Long-term complications of T2DM include diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, and macrovascular problems.
Diabetic retinopathy is one of the most common causes of blindness in the working age population in Ireland. Up to 10 per cent of people with diabetes are at risk of sight-threatening retinopathy. Diabetic retinopathy may have no obvious symptoms in its early stages, but when caught early, treatment is effective at reducing or preventing damage to sight.10 The national diabetic retinopathy screening programme,

Diabetic RetinaScreen, has been providing free retinal screening to all diabetes patients in Ireland over the age of 12 years since 2013.18 Since the programme’s launch, prevalent, undiagnosed, and untreated diabetic retinopathy and maculopathy have been successfully identified and referred for evidence-based treatments at the programme’s seven treatment centres before any significant visual symptoms occur. Adding patients to the register to be screened is easy, and can be done by a patient’s GP, GPN, or allied health professionals using the free phone
number: 1800-454-555 or email: info@ diabeticretinascreen.ie. Full screening information is available at www.diabeticretinascreen.ie.
Diabetic nephropathy is a significant cause of chronic kidney disease and endstage renal failure globally. If untreated, diabetic nephropathy can lead to impaired kidney function, dialysis and/or kidney transplant. Diabetic nephropathy is identified when eGFR is <60mL/ min/1.73m² and albuminuria >30mg/g creatinine.8,14 Annual assessment (at least) with urine ACR, serum creatinine and
eGFR is recommended.18

Diabetic neuropathy is the most common complication associated with diabetes mellitus. Diabetes causes a broad spectrum of neuropathic complications, including peripheral, autonomic, proximal, and focal. Diabetic peripheral neuropathy (DPN) is the most common form of nerve damage, and it most often affects the nerves to the hands and feet. DPN leads to distressing and expensive clinical sequelae such as foot ulceration, leg amputation, and neuropathic pain. DPN is often diagnosed late when irreversible nerve injury has occurred, and its first presentation may be with a diabetic foot ulcer.1 DPN may be present at time of diagnosis in more than 10 per cent of patients and may affect up to 50 per cent of patients with longstanding diabetes. In 50 per cent of cases, DPN may be asymptomatic, but for 16-to-26 per cent of patients with diabetes the neuropathy is painful. Patients should be examined for DPN from time of diagnosis.18

Macrovascular: T2DM can also affect the large blood vessels, causing plaque to build up, leading to a heart attack, stroke and peripheral vascular disease. Cardiovascular disease (CVD) is the leading cause ( 70 per cent) of death in people withT2DM. People with diabetes have a four-fold greater risk for having a CVD event than people without diabetes after controlling for traditional risk factors for CVD such as age, obesity, tobacco use, dyslipidaemia, and hypertension.16
Prevention and patient education
Patient education and effective lifestyle modifications including weight loss, and adoption of a healthy diet together with increased physical activity are the cornerstones for the prevention of T2DM. Emphasis must be placed on promoting a healthier lifestyle and finding solutions for increased adherence and compliance, especially for high-risk individuals.
Think Once-Weekly Ozempic for
People with Type 2 Diabetes who have Atherosclerotic Cardiovascular Disease1-3
Think Once-Weekly for People with who have Atherosclerotic Cardiovascular
Ozempic®
significant 26% risk reduction of MACE people with type 2 diabetes
1,2,§


39% relative reduction in non-fatal stroke.
superior glucose and weight-loss across all head-to-head clinical program.1-9,†,‡
* 26% CV risk reduction in patients with type 2 diabetes and high CV risk, compared to placebo, in addition to standard treatment.
# Major Adverse Cardiovascular Events
26% CV risk reduction in patients with type 2 diabetes and high CV across SUSTAIN trials, which included
† Results apply to Ozempic® across SUSTAIN trials, which included placebo, sitagliptin, dulaglutide, canagliflozin, exenatide PR and glargine U100.1-9
§ p=0.02 for superiority
‡ p<0.05
Ozempic® is recommended by the ADA/EASD Consensus Report for people with type 2 diabetes who have established atherosclerotic cardiovascular disease10
is recommended by Consensus Report for people diabetes who have established
atherosclerotic cardiovascular
PR = Prolonged Release; ADA = American Diabetes Association; EASD = European Association for the Study of Diabetes.
SUSTAIN = Semaglutide Unabated Sustainability in treatment of Type 2 Diabetes.
*SUSTAIN was the phase 3 clinical trial programme investigating the effects of once weekly semaglutide versus other anti-diabetic agents.
ABBREVIATED PRESCRIBING INFORMATION
Ozempic®t semaglutide



sulfonylurea or insulin, patients should be advised to take precautions while driving and using machines. childbearing potential are recommended to use contraception when Should not be used during pregnancy or breast-feeding. Discontinue planned pregnancy. Effect on fertility unknown. (≥1/10): (≥1/100 to <1/10):
decreased appetite, dizziness, diabetic retinopathy complications, abdominal distension, constipation, dyspepsia, gastritis, gastroeructation, flatulence, cholelithiasis, fatigue, increased lipase, decreased. rate, acute pancreatitis, injection site reactions. reaction. Not known (cannot be estimated from available data): should be consulted for a full list of side effects. pen EU/1/17/1251/002. Ozempic
4 disposable NovoFine information, please refer to the SmPC which is available on www. infoireland@novonordisk.com or from the Clinical, Medical Novo Nordisk Limited, 1st Floor, Block A, The Crescent Building, Santry, Dublin 9.
Please refer to the Summary of Product Characteristics (SmPC) before prescribing. Ozempic® 0.25 mg solution for injection in pre-filled pen. Ozempic® 0.5 mg solution for injection in pre-filled pen. Ozempic® 1 mg solution for injection in pre-filled pen. One ml of solution contains 1.34 mg of semaglutide (human glucagon-like peptide-1 (GLP-1) analogue). Indication: Ozempic® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1 of the Ozempic® SmPC. Posology and administration: Administered once weekly at any time of the day, with or without meals. Injected subcutaneously in the abdomen, thigh or upper arm. Starting dose: 0.25 mg once weekly. After 4 weeks the dose should be increased to 0.5 mg once weekly. After at least 4 weeks with a dose of 0.5 mg once weekly, the dose can be increased to 1 mg once weekly to further improve glycaemic control. When Ozempic® is added to existing metformin and/ or thiazolidinedione therapy or to an SGLT2 inhibitor, the current dose of metformin and/ or thiazolidinedione or SGLT2 inhibitor can be continued unchanged. When Ozempic® is added to a sulfonylurea or insulin, a reduction in dose of sulfonylurea or insulin should be considered to reduce the risk of hypoglycaemia. Blood glucose self-monitoring is necessary to adjust the dose of sulfonylurea and insulin, particularly when Ozempic® is started and insulin is reduced. A stepwise approach to insulin reduction is recommended. Children: No data available. Elderly: No dose adjustment required, therapeutic experience in patients age ≥75 is limited. Renal impairment: No dose adjustment is required for patients with mild, moderate
or severe renal impairment. Experience in patients with severe renal impairment is limited. Not recommended for use in patients with end-stage renal disease. Hepatic impairment: No dose adjustment is required for patients with hepatic impairment. Experience with severe hepatic impairment is limited. Caution should be exercised when treating these patients with semaglutide. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Special warnings and precautions for use: Should not be used in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis. Not a substitute for insulin. Diabetic ketoacidosis has been reported in insulin-dependent patients whom had rapid discontinuation or dose reduction of insulin. There is no experience in patients with congestive heart failure NYHA class IV and is therefore not recommended in these patients. Use of GLP-1 receptor agonists may be associated with gastrointestinal adverse reactions. This should be considered when treating patients with impaired renal function as nausea, vomiting, and diarrhoea may cause dehydration which could cause a deterioration of renal function. Acute pancreatitis has been observed with the use of GLP-1 receptor agonists. Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, semaglutide should be discontinued; if confirmed, semaglutide should not be restarted. Caution should be exercised in patients with a history of pancreatitis. Use of semaglutide in combination with a sulfonylurea or insulin may have an increased risk of hypoglycaemia, therefore consider reducing the dose of sulfonylurea or insulin when initiating treatment with Ozempic®. In patients with diabetic retinopathy treated with insulin and semaglutide, an increased risk of developing diabetic retinopathy complications has been observed. Caution should be exercised when using semaglutide in patients with diabetic retinopathy treated with insulin. These patients should be monitored closely and treated according to clinical guidelines. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy, but other mechanisms cannot be excluded. When semaglutide is used
in combination with a sulfonylurea or insulin, patients should be advised to take precautions to avoid hypoglycaemia while driving and using machines. Fertility, pregnancy and lactation: Women of childbearing potential are recommended to use contraception when treated with semaglutide. Should not be used during pregnancy or breast-feeding. Discontinue at least 2 months before a planned pregnancy. Effect on fertility unknown. Undesirable effects: Very common (≥1/10): Hypoglycaemia when used with insulin or sulfonylurea, nausea, diarrhoea. Common (≥1/100 to <1/10): Hypoglycaemia when used with other oral antidiabetic medications, decreased appetite, dizziness, diabetic retinopathy complications, vomiting, abdominal pain, abdominal distension, constipation, dyspepsia, gastritis, gastrooesophageal reflux disease, eructation, flatulence, cholelithiasis, fatigue, increased lipase, increased amylase, weight decreased. Uncommon (≥1/1,000 to <1/100): Hypersensitivity, dysgeusia, increased heart rate, acute pancreatitis, injection site reactions. Rare (≥1/10,000 to <1/1,000): Anaphylactic reaction. Not known (cannot be estimated from available data): Angioedema. The SmPC should be consulted for a full list of side effects. MA numbers: Ozempic® 0.25 mg pre-filled pen EU/1/17/1251/002. Ozempic® 0.5 mg pre-filled pen EU/1/17/1251/003. Ozempic® 1 mg pre-filled pen EU/1/17/1251/005. Each pre-filled pen delivers 4 doses and includes 4 disposable NovoFine® Plus needles. Legal Category: POM. For complete prescribing information, please refer to the SmPC which is available on www. medicines.ie or by email from infoireland@novonordisk.com or from the Clinical, Medical and Regulatory Department, Novo Nordisk Limited, 1st Floor, Block A, The Crescent Building, Northwood Business Park, Santry, Dublin 9. Date last revised: March 2021.
reported to the Health Products Regulatory Authority. Information reporting is available at www.hpra.ie. Adverse events should also be Tel: 1850 665 665 or complaintireland@novonordisk.com
tAdverse events should be reported to the Health Products Regulatory Authority. Information about adverse event reporting is available at www.hpra.ie. Adverse events should also be reported to Novo Nordisk on Tel: 1850 665 665 or complaintireland@novonordisk.com
Consoli A, 834–44. glargine as add-on to metformin (with or without sulf monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a add-on to metformin, thiazolidinediones, or both, in patients with type open-label, phase 3b trial. 43(2):487-493.
Randomized Clinical Trial. Diabetes Care 2018;41:258-266. 6. Aroda VR et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulinnaive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017;5: 355–66. 7. Sorli C et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251–60. 8. Ahrén B et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5: 341–54. 9. Pratley RE et al. Semaglutide versus dulaglutide once-weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6275-286. 10.Buse JB et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020 Feb; 43(2):487-493.
Semaglutide
Once weekly Semaglutide vs Canagliflozin versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, Lancet Diabetes 2017; 5:
2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report
Ozempic® is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise • as monotherapy when metformin is considered inappropriate due to intolerance or contraindications • in addition to other medicinal products for the treatment of diabetes. For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. of the SmPC.1 tThis medicinal product is subject to additional monitoring. This will allow quick identification of new safety information.
or severe Not dose hepatic semaglutide. excipients. with for insulin. rapid congestive Use should and Acute be informed semaglutide Caution in combination therefore Ozempic increased should insulin. Rapid diabetic is indicated for the treatment of adults with insufficiently controlled type 2 diabetes metformin is considered inappropriate due to intolerance or contraindications • in addition results with respect to combinations, effects on glycaemic control and cardiovascular events, This medicinal product is subject to additional monitoring. This will allow quick identification
Novo Nordisk Limited, First Floor, Block A, The Crescent Building, Northwood Business Park, Santry, Dublin 9, D09 X8W3, Ireland.
www.novonordisk.ie
provided a significant 26% risk reduction of MACE# in people with type 2 diabetes* and cardiovascular disease.1,2,§
This includes a 39% relative risk reduction in non-fatal stroke.1,2,‡
Ozempic® also has superior blood glucose and weight-loss efficacy across all head-to-head clinical trials in the SUSTAIN program.1-9,†,‡
Diabetes SMART is a new free interactive online education course developed by Diabetes Ireland, for people diagnosed with T2DM. The Diabetes SMART programme contains six interactive modules, covering topics that explain what diabetes is, understanding the key medical information such as blood glucose levels, managing illness, and providing tips on healthy eating and getting active. The programme was developed by diabetes healthcare professionals, and the resource will give people with T2DM the knowledge and accessible tools to learn how to manage their condition and protect their future health.1
The HSE also provides a number of free educational resources and support courses to diabetes patients, both online and in person. See www.hse.ie/services/diabetessupport-courses/diabetes-support-courses. html for more information.
Outlook
While there is still no cure for T2DM, several drugs are in their developmental stages. Perhaps the most anticipated is the glucagon-like peptide-1 (GLP-1) receptor agonists, which induce insulin production while also suppressing the secretion of glucagon.7
Imeglimin, a drug being developed by the French company Poxel, has shown great promise in a phase 3 clinical trial in Japan. Damage to the mitochondria, the structures that generate energy within cells, plays a key role in the progression of metabolic diseases, and imeglimin protects mitochondria from damage. With this unique method of action, imeglimin has the potential to treat T2DM by acting in three organs at once: The pancreas, the liver, and the muscles to reduce blood glucose levels.16
Adjustments to dietary nutrient composition, insulin-secreting cell implants, bariatric surgery, and agents primarily designed to suppress appetite and reduce adiposity, will also greatly contribute to the future management of T2DM.
REFERENCES
1. Diabetes Ireland (2022). Diabetes prevalence in ireland. Diabetes Ireland. Available at: www.diabetes.ie/aboutus/diabetes-in-ireland/
2. Leahy S, O'Halloran AM, O'Leary N, Healy M, McCormack M, Kenny RA, O'Connell J. Prevalence and correlates of diagnosed and undiagnosed type 2 diabetes mellitus and pre-diabetes in older adults: Findings from the Irish Longitudinal Study on Ageing (TILDA). Diabetes Res Clin Pract. 2015 Dec;110(3):241-9. doi: 10.1016/j. diabres.2015.10.015
3. International Diabetes Federation Diabetes Atlas (2021), Available at: https://diabetesatlas.org/
4. Nolan J, O’Halloran D, McKenna T, Firth R, Redmond S. (2006). CODEIRE. The cost of treating type 2 diabetes. Available at: http:// archive.imj.ie/ViewArticleDetails. aspx?ArticleID=1508
5. Bellou V, Belbasis L, Tzoulaki I, Evangelo E. (2017). Risk factors for type 2 diabetes mellitus: An exposurewide umbrella review of metaanalyses. Available at: https://doi. org/10.1371/journal.pone.0194127
6. Goyal R, Jialal I. Diabetes mellitus type 2. In StatPearls Publishing; 2022 Jan. Available from: www.ncbi.nlm.nih. gov/books/NBK513253/
7. Lam M. (2019). Diagnosis and management of type 2 diabetes mellitus. The Pharmaceutical Journal Vol 303; No 7929; 303. doi: 10.1211/ PJ.2019.20206770
8. International Diabetes Federation. (2017). IDF clinical practice recommendations for managing type 2 diabetes in primary care. Available at: www.idf.org/e-library/ guidelines/128-idf-clinical-practicerecommendations-for-managing-type2-diabetes-in-primary-care.html
9. World Health Organisation. (2011). Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: Abbreviated report of a WHO Consultation. Available at: www.who. int/diabetes/publications/report-
hba1c_2011.pdf?ua=1
10. ICGP (2016). A practical guide to integrated type 2 diabetes care. Irish College of General Practitioners. Ireland
11. Goyal R, Jialal I, Castano M. (2022). Diabetes mellitus type 2 (nursing). In StatPearls Publishing; 2022 Jan. Available from: www.ncbi. nlm.nih.gov/books/NBK568737/
12. National Institute for Health and Care Excellence. Type 2 diabetes in adults: Management. NICE guideline [NG28]. 2019. Available at: www.nice. org.uk/guidance/NG28
13. HSE. (2021). Diabetic foot model of care. Health Service Executive. Available at: www.hse.ie/eng/about/ who/cspd/ncps/diabetes/moc/ diabetic-foot-model-of-care-2021.pdf
14. Lim A. (2014). Diabetic nephropathy - complications and treatment. International Journal of Nephrology and Renovascular Disease, 7, 361–381. doi: org/10.2147/ IJNRD.S40172
15. Yang H, Sloan G, Ye Y, et al. (2020). New perspective in diabetic neuropathy: From the periphery to the brain, a call for early detection, and precision medicine. Front Endocrinol, 17 January 2020. doi: org/10.3389/ fendo.2019.00929
16. Cade WT. (2018). Diabetesrelated microvascular and macrovascular diseases in the physical therapy setting. Physical Therapy, 88(11), 1322–1335. doi: org/10.2522/ptj.20180008
17. Smith J. (2019). French first-inclass diabetes drug nails phase III trial. Labiotech.EU. Available at: www. labiotech.eu/trends-news/poxel-type2-diabetes-japan/
18. HSE. (2018). Model of integrated care for patients with type 2 diabetes. A guide for healthcare professionals (Clinical Management Guidelines). Available at: www.hse.ie/eng/about/ who/cspd/ncps/diabetes/moc/ model-of-integrated-care-type-2diabetes-2018.pdf
Prescribing Information:
For the treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years1 ®

Toujeo (insulin glargine 300 units/ml)
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection.
Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and
Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapid-acting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit to unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%). Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients. Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an
References: 1. Toujeo® Summary of Product Characteristics
Date of preparation: September 2020 | MAT-IE-2001062 (v1.0)
unaffected area has been reported to result in hypoglycaemia. Blood glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar pre-filled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There is no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Legal Category: POM. Marketing Authorisation Number: SoloStar 3 Pen pack: EU/1/00/133/034, DoubleStar EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2020.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
Q1. Diabetes mellitus is the most common chronic metabolic disease and a major source of morbidity and mortality. Type 1 diabetes mellitus is the most prevalent form accounting for around 90 per cent of cases worldwide.
True or false?
Q2. The prevalence of diabetes worldwide is growing at an alarming rate, and is predicted to rise to 643 million by 2030, and 784 million by 2045. The diabetes epidemic is unfolding because of increasing obesity rates, sedentary lifestyles and an ageing population.
True or false?
Q3. T2DM is an insulindeficient condition with associated alpha-cell dysfunction.
True or false?
TRUE/FALSE QUESTIONS
Q4. T2DM occurs when blood glucose levels are too low (hypoglycaemia) due to insufficient insulin production, or when the insulin that is produced by the pancreas is not working effectively.
True or false?
Q5. Obesity and T2DM are closely linked and are increasing in prevalence worldwide. Both chronic conditions have a multisystem impact and are associated with increased mortality and cardiovascular risk.
True or false?
Q6. Symptoms of T2DM originate from persistent hypoglycaemia and the increased ability to use glucose as fuel, and include polycythaemia, rash, polydipsia, fatigue, and weight gain. A person with diabetes may also experience
other symptoms such as blurred vision, increased sensations, or pain in the hands and feet, along with recurrent genitourinary infections.
True or false?
Q7. T2DM has a long pre-clinical phase and may be asymptomatic until well after longterm microvascular and macrovascular complications have occurred. T2DM can be detected before the onset of symptoms and clinical signs by identifying people who are at risk and performing diagnostic testing.
True or false?
Q8. Diagnosis of T2DM can be made when fasting plasma glucose is ≥6.0mmol/L or random plasma glucose is ≥10.1mmol/L in the presence of
symptoms such as frequent urination, thirst and unexplained weight gain. The oral glucose tolerance test (OGTT) can also be used as a diagnostic tool, where a diagnosis is made if a plasma glucose level of ≥9.1mmol/L is measured two hours after the ingestion of a 75g glucose solution.
True or false?
Q9. A HbA1c result of ≥48mmol/mol (6.5 per cent) is recommended as the threshold for diagnosing diabetes.
True or false?
Q10. All patients with diabetes should be screened every two years to identify their foot ulcer risk status.
True or false?
COMPLETE THIS MODULE ONLINE
Check your answers against the latest module on nursecpd.ie Successful completion of this module will earn you 2 CPD credits

IRISH SOCIETY FOR RHEUMATOLOGY SPRING MEETING, SLIGO, 19-20 MAY 2022

Tackling waiting lists and obtaining off-site facilities to treat patients is top of the agenda for the Model of Care for Rheumatology on emerging from the pandemic, according to HSE National Clinical Lead for Rheumatology, Prof David Kane.
Prof Kane, Consultant Rheumatologist at Tallaght University Hospital, Dublin, told NiGP that during the Covid-19 pandemic, waiting lists didn’t grow significantly and became “fairly static”. “We’ve managed to maintain it by moving to virtual technology to keep our clinical appointments ticking over,” he said. “But we do have to see our patients again.”
Furthermore, the number of referrals is now growing again, but capacity is not, Prof Kane pointed out.

In the Model of Care for Rheumatology in Ireland, published in 2018, one of the objectives of the programme for access to secondary care was to reduce wait times for referrals to rheumatology.
“It’s not been an easy couple of years. What we want to address now is getting back to normal business, optimising care for our patients and getting the waiting list down,” Prof Kane added. “We need to improve capacity, change a little bit about the way we work, and move forward with the multidisciplinary strands of our
programme to deliver care through nonrheumatologists as well.”
Prof Kane is a firm advocate for taking a multidisciplinary approach to rheumatology treatment and management. “To date we have 184,000 people taken off the orthopaedic and rheumatology waiting list since the programme introduced the musculoskeletal (MSK) physio triage programme,” he said.

The National MSK Physiotherapy Triage Initiative is delivered by advanced practice physiotherapists who see referred MSK patients and triage referrals to consultant orthopaedic surgeons and consultant rheumatologists. “We’re
now trying a similar initiative with occupational therapy,” he said.
Prof Kane believes rheumatology “probably” needs an outpatient building programme. “The other impact of the pandemic is that we’ve been to some extent squeezed-off the acute hospital sites because acute medicine was a more pressing need,” said Prof Kane. “And what we realised is that we need probably a big investment in rheumatology units nationally to build… places for us to see patients because most of us are finding it hard to get space to see patients in the existing acute hospital sites.”
He said they are looking for simple, off-site facilities and do not require any “highly technical working space”.
During his presentation at the Irish Society for Rheumatology (ISR) 2022 Spring Meeting, which took place in the Sligo Park Hotel on 19 and 20 May, Prof Kane discussed waiting times and expanding HSE rheumatologist posts from 42 two years ago to 52 this year, aiming for an overall expansion to 80 posts by 2028.
He also discussed how the Clinical Programme is working on restructuring “how we manage inflammatory arthritis and trying to create better pathways for people”.
Waiting lists and outpatient facilities need to be tackled as a priority in rheumatology
Prof David Kane
Issues in accessing biologics for lupus patients in Ireland
Irish rheumatologists are struggling to access two recently-approved biological drugs to treat lupus, the ISR 2022 Spring Meeting heard.

Dr Eoghan McCarthy, Consultant Rheumatologist at Beaumont Hospital, Dublin, gave an update on the use of biologic drugs in systemic lupus erythematosus (SLE), the most common type of lupus.
The monoclonal antibody drug belimumab, which can be used to treat patients with SLE over the age of five years and adults with lupus nephritis, has been approved for use in the EU since 2011. Another monoclonal antibody, anifrolumab, which is given to SLE patients with autoantibodies who still have moderate-to-severe disease despite standard treatment, was approved in the EU in February 2022. Neither drug are currently available in Ireland, however, he said.
“It is disease activity that you need to try and get under control as quickly as possible because those are the things that will cause damage,”
Dr McCarthy told the Meeting. “But over time… the treatments that you’re actually exposing these patients to is what causes the accumulation of damage.
“So, anything you can do to reduce damage, and therefore anything you can do to reduce the accumulative burden of steroid exposure to these patients, and reducing disease activity in a rapid manner will be associated with improved outcomes for our patients. And that’s the desperation that we all have as rheumatologists… how do you
manage to do that without having to rely on such toxic medications.”
Belimumab was approved as an add-on therapy for SLE. “I think it is important that, actually, it looks like it’s a reasonably safe drug in terms of standard of care,” said Dr McCarthy. The drug is approved for patients with active, antibody-positive SLE with a high degree of disease activity despite already receiving standard treatment.
Anifrolumab, approved only months before the ISR Spring Meeting, “looks to be quite a promising drug”, he commented. “There’s a rationale that interferon inhibitors may… reduce SLE activity.”
In SLE, a protein called type I interferon is involved in causing the immune system to attack normal cells and tissues. Anifrolumab works by blocking the action of this protein and then reduces inflammation and organ damage.
As part of showing the efficacy of biologics in the real world, Dr McCarthy spoke about the British Isles Lupus Assessment Group Biologic Register (BILAG-BR), a study underway in UK hospitals to assess the safety and effectiveness of biologic and biosimilar treatment for SLE. “The fact that we are demonstrating efficacy in the type of patients that are walking into your clinic is important,” he said.
“For patients, I think it’s really important to understand the application and the safety of these drugs in the real-world setting, particularly as more and more of them come through,” he added, “because all our patients are going to be receiving
a kind of belimumab or anifrolumab on the background of having probably multiple courses of [other treatments].”
Dr McCarthy told NiGP that the main takeaway from his talk at the meeting was that: “We as treating clinicians in Ireland are unable to access either belimumab or anifrolumab at present.”
In response to queries from NiGP as to why this was the case, the HSE said it had not received the necessary applications for the drugs from their manufacturers.
“Pharmaceutical companies are required to submit formal applications to the HSE if they wish their medicines to be added to the list of reimbursable items/funded via hospitals,” a spokesperson for the Executive said.
The Corporate Pharmaceutical Unit (CPU) within the HSE is responsible for accepting and considering pricing and reimbursement applications from the pharmaceutical industry. “As of 30 May 2022, the HSE CPU can confirm that a pricing and reimbursement application has not been submitted by the pharmaceutical company (AstraZeneca) for anifrolumab.”
Regarding belimumab, the HSE Drugs Group, responsible for recommendations on pricing and reimbursement of medicines, was “unable to support the pricing approval of this medicine in 2013 due to the absence of a Health Technology Assessment (HTA)”. The HSE spokesperson said, to date, GlaxoSmithKline Pharmaceuticals has not submitted a HTA dossier for belimumab to the National Centre for Pharmacoeconomics for assessment.

































 Rx Methofill® by brand.
Rx Methofill® by brand.
NEW RESOURCES ON MENOPAUSE
In response to increased demand for more information in Ireland on the topic of the menopause, a list of patient resources on menopause has been assembled by the ICGP’s Director of Women’s Health, Dr Nóirín O’Herlihy and the College’s first GP Clinical Lead in Women’s Health, Dr Ciara McCarthy, which are now available on the ICGP’s website, www.icgpnews.ie/ menopause-patient-information/.
The resources include five short videos to give some general information about menopause, what it is, how it is diagnosed, and what to expect from its treatment.
ICGP video content on menopause
1. What is menopause?
Dr Ciara McCarthy explains what menopause is and the symptoms women may experience.
2. Talking to your GP about menopause
Dr Nóirín O’Herlihy explains what to expect at the first GP visit to discuss the menopause. The consultation will include symptoms the woman is having, eg, hot flushes, sleep disturbance, mood changes, and night sweats.
3. The risks and benefits of HRT
Dr Nóirín O’Herlihy explains the risks and benefits of HRT, and if HRT is a suitable option. For example, HRT may not be suitable for women if they have had a recent heart attack or stroke, or hormone-sensitive cancer. HRT benefits include symptom relief, and the risks are quite low. The sideeffects of HRT are described.
4. The different types of HRT
Dr Ciara McCarthy explains the types and formulations of HRT available. The GP will discuss whether the woman wishes to use HRT and which is the best type for them such as patches, capsules, or vaginal.
5. Lifestyle advice in menopause and perimenopause
Dr Ciara McCarthy explains that menopause can be a significant life change, but making some simple lifestyle changes can make a positive difference, eg, aerobic and weightbearing exercise, and dietary changes to ensure women are getting enough calcium and vitamin D.
New women’s health services
Minister for Health Stephen Donnelly recently announced €2.5 million in funding for priority areas within the Women’s Health Action Plan, including the menopause.
The Women’s Health Fund (€10m) will ringfence €2.5 million for the National Women and Infants Health Programme in the HSE to accelerate service delivery in four critical areas in 2022.
Menopause: The Women’s Health Action Plan 2022-23 committed to developing four specialist menopause clinics nationally in 2022; this further investment will support two additional specialist menopause clinics, bringing a total of six such clinics nationwide for women who require complex, specialist care.
Postnatal care: The Women’s Health Action Plan 2022-23 committed
to developing a new more holistic model for supporting women in the weeks after giving birth, this investment will support two additional community-based postnatal hubs for women, bringing a total of four hubs nationwide in 2022.
Endometriosis: The Women’s Health Action Plan 2022-23 committed to supporting the ongoing development of two supra-regional specialist centres for complex care for endometriosis for the first-time. This latest investment will create a new tier of additional support at hospital level, by investing in resources for six additional interdisciplinary teams to support holistic treatment of endometriosis within each of the hospital networks.
Targeted support for marginalised women: The Women’s Health Action Plan 2022-23 put a clear focus on increasing supports for marginalised women and groups that face multiple disadvantages. This investment will support the implementation of additional medical social work resources across the six maternity networks, significantly enhancing this critical support at what can be a vulnerable time for many women.
Dr Cliona Murphy, Clinical Lead for the National Women and Infants Health Programme in the HSE, has warmly welcomed this allocation saying: “The Minister has encouraged us to be ambitious in our work on women’s health. To be in a position mid-year, through this allocation, to expand and accelerate our already ambitious work programme is really satisfying because we know first-hand the difference this will makes to the lives of women right around the country.”
Dryness and sensitive skin and membranes
• Scientifically documented
• Suited for vegetarians and vegans
• With vitamin A that supports normal skin, vision and mucous membranes
Vaginal dryness, dry eyes and dry mouth are issues women typically experience around and after menopause.
Vaginal dryness, dry eyes and dry mouth are issues women typically experience around and after menopause.


BioActive Omega 7 Pharma Nord is a formula developed to help maintain healthy and well hydrated mucosa at this stage of life.
BioActive Omega 7 Pharma Nord is a formula developed to help maintain healthy and well hydrated mucosa at this stage of life.
BioActive Omega 7 Pharma Nord contains the SBA24 extract that is made from both the berries and seeds of Sea Buckthorn to ensure the widest spectrum of beneficial nutrients.
BioActive Omega 7 Pharma Nord contains the SBA24 extract that is made from both the berries and seeds of Sea Buckthorn to ensure the widest spectrum of beneficial nutrients.

Sea Buckthorn is one of nature’s richest sources of vitamin A, a nutrient that is best known for its ability to maintain normal skin, vision, and mucous membranes.
Sea Buckthorn is one of nature’s richest sources of vitamin A, a nutrient that is best known for its ability to maintain normal skin, vision, and mucous membranes.
ERECTILE DYSFUNCTION
ERECTILE DYSFUNCTION IS A COMMON ISSUE IN MEN, PARTICULARLY OLDER MEN WITH COMORBIDITIES
Erectile dysfunction (ED), the inability to achieve or maintain an erection for satisfactory sexual performance, affects a considerable proportion of men at least occasionally and can have a substantial negative impact on quality-of-life.1 It is estimated that at least 150 million men globally have ED. It is difficult to obtain accurate values for the true prevalence of ED, however, as many patients fail to seek medical attention, and many clinicians are reluctant to ask patients about their sexual health.
ED is generally treatable, however, if left untreated, ED can be a source of severe emotional stress for both the man and their partner.
ED can be a symptom of a wide range of underlying pathologies, and is often an under-recognised but important cardiovascular risk factor.1 Owing to its strong association with metabolic syndrome and cardiovascular disease (CVD), cardiac assessment is warranted in men with symptoms of ED. 2
Aetiology

Although most men will experience periodic episodes of ED, it tends to become more frequent with advancing age. Many factors can contribute to sexual dysfunction in older men, including physical and psychological conditions, comorbidities and polypharmacy. Aspects of an ageing man’s lifestyle behaviour and androgen
deficiency, most often decreasing testosterone levels, can affect sexual function.1,6 Studies have shown that the percentage of men who engage in some form of sexual activity decreases from 73 per cent in men aged 57-64 years to 26 per cent for men aged 75-85 years. The aetiology for this decline in male sexual activity is multifactorial, and is in part related to female partners’ menopause at approximately 52 years of age, leading to a significant decline in female libido and desire to engage in sexual activity.7
age.11 Another study in 2016 concluded that 22.1 per cent of men aged under 40 years of age had low (<21) Sexual Health Inventory for Men (SHIM) scores.13
In the past, ED was almost always considered a psychogenic disorder. However, evidence now suggests that more than 80 per cent of cases have an organic aetiology. 2 While most patients with ED have organic disease, some do have a primary psychological cause, particularly younger men. Even when ED is organic in nature, there are almost always psychological consequences regarding relationship issues, cultural norms and expectations, loss of self-esteem, shame, and anxiety and depression related to sexual performance. 1
While ED is associated with ageing, many studies and large scale surveys have concluded that ED is also a major health concern among young men.11,12,13
One study in 2013 reported that one-infour men seeking medical help for ED in the real-life setting is <40 years of
ED is multidimensional in nature, and can be broadly divided into endocrine and non-endocrine causes. The condition can be caused by any disease process which affects penile arteries, nerves, hormone levels, smooth muscle tissue, corporal endothelium, or tunica albuginea. It is closely related to CVD, diabetes mellitus, hyperlipidaemia, hypertension, and endothelial dysfunction. ED and vascular disease are thought to be linked at the level of the endothelium. Endothelial dysfunction results in the inability of smooth muscle cells lining the arterioles to relax and prevents vasodilatation. 3 The endothelial cell is known to affect vascular tone and impact the process of atherosclerosis
OWING TO ITS STRONG ASSOCIATION WITH METABOLIC SYNDROME AND CARDIOVASCULAR DISEASE (CVD), CARDIAC ASSESSMENT IS WARRANTED IN MEN WITH SYMPTOMS OF ED
and impacting ED, CVD and peripheral vascular disease. 6
CVD and hypertension are very significant risk factors for ED. CVD causes a narrowing and hardening of the arteries, leading to reduced blood flow to the corporal bodies, which is essential for achieving an erection. 6 Both CVD and ED involve endothelial cell dysfunction in their pathophysiology. Besides CVD, there are strong correlations between ED and hyperlipidaemia, diabetes, hypogonadism, obesity,
smoking, alcoholism, benign prostatic hyperplasia (BPH) with lower urinary symptoms (LUTS), depression, and premature ejaculation.1
Diabetes is a common aetiology of sexual dysfunction, because it can affect both the blood vessels and the nerves that supply the penis. Men with diabetes are four times more likely to experience ED and on average experience it 15 years earlier than men without diabetes. Obesity is also correlated to the development of
several types of dysfunction, including a decrease in sex drive and an increase in episodes of ED. 6

Neurogenic ED is caused by a deficit in nerve signalling to the corpora cavernosa . Such deficits can be secondary to spinal cord injury, multiple sclerosis, Parkinson’s disease, lumbar disc disease, traumatic brain injury, radical pelvic surgery, and diabetes. 2
Men being treated for prostate cancer with treatments such as radical prostatectomy, radiation therapy,
Source: www.urologypartners.co.uk/repository/originals/The_International_Index_of_Erectile_Function.pdf
or the use of luteinising hormonereleasing hormone (LHRH) agonists and antagonists, often experience ED. 6
The International Index of Erectile Function (IIEF-5) Questionnaire
The IIEF 4,5 is a multidimensional validated questionnaire with 15 questions in the five domains of sexual function (erectile and orgasmic functions, sexual desire, satisfaction with intercourse, and overall sexual satisfaction), and there is also an abbreviated format of five questions in the SHIM. 5 The severity of ED is described as mild, moderate or severe, according to the IIEF-5 questionnaire, with a score of 1-7 indicating severe; 8-11 moderate; 12-16 mild–moderate; 17-21 mild; and 22-25 no ED. 2,4
Investigations and diagnosis
A thorough medical history, detailed sexual history, and physical examination are required before commencing treatment or further investigations for ED. It is important to distinguish between psychological and organic causes of ED, as well as to ensure that the patient has ED and not another disorder. History that points towards a psychological aetiology include sudden onset of ED, especially if it is related to a new partner or a major life-changing event, situational ED, normal erections with masturbation or a different partner, presence of morning erections, and high daily variability in erectile rigidity.1,6
The main differential diagnoses for ED are hypogonadism, loss of libido, depression with low mood, and other psychological conditions. It may also be the first manifestation of diabetes or CVD as well as depression. It is important to differentiate between true ED and other sexual disorders such as premature ejaculation, and this is usually assessed by obtaining a good sexual history. 1,2
A complete medication list including
supplements should be checked with the patient. Numerous medications are listed with ED and/or a decreased libido as a side-effect. Antidepressants, particularly the SSRIs such as citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline, can contribute to ED. 6 Prescription drugs that can cause ED include hydrochlorothiazide, cimetidine, ketoconazole, spironolactone, sympathetic blockers, thiazide diuretics, and other antihypertensives. ACE inhibitors and calcium channel blockers are the least likely to cause ED. Beta-blockers are only a minor contributor, while alpha-blockers can improve erectile function.1,2 Vascular risk factors such as hypertension and diabetes and lifestyle factors such as smoking, activity level, alcohol intake, and the use of any recreational drugs should be assessed. 1 A full general and cardiovascular examination should be undertaken,
as ED can be the first symptom of underlying vascular disease. Peripheral pulses should be checked and blood pressure measured. The genitalia should be carefully inspected for hypogonadism, signs of infection, the presence of penile fibrosis or plaques, and phimosis. Hair distribution, breast size (gynaecomastia), and a detailed neurological examination are important. The cremasteric reflex should be evaluated. A normal reflex is retraction or elevation of the ipsilateral testicle. This reflex will be normal if the thoracolumbar erection centre is intact.1,2
Investigative blood tests include a full blood count, electrolytes, renal and liver function tests, HbA1c to screen for diabetes mellitus, and a lipid profile. Testosterone levels are checked and a morning testosterone level is recommended, especially if

there are symptoms suggestive of hypogonadism such as loss of sexual desire or testicular atrophy on physical examination. Thyroid function (TSH) may also be measured. Other blood tests can include LH and prolactin if hypogonadism is found, and sickle cell in African/Caribbean patients. 1,2
Treatment and management
Initial treatment for ED is based on lifestyle modification followed by firstline therapies using PDE5 inhibitors and vacuum erection devices (VEDs). Second-line therapies consist of an intraurethral suppository (IUS) of prostaglandin E1 (alprostadil) and intracavernosal injection (ICI) with vasoactive substances. Surgical intervention is usually reserved as the final option after conservative options have been discussed or attempted. 6
The role of testosterone replacement therapy (TRT) as a potential to improve erectile function in ED remains an issue for clinicians who are comfortable treating androgen deficiency. Androgens are known to have a significant impact on the function of the smooth musculature within the corpus spongiosum. Testosterone supplementation is more effective as a treatment for low libido than for ED. For most men with both ED and hypogonadism, oral PDE5 inhibitors alone are recommended as the initial therapy. Testosterone supplementation is reasonable in men with proven hypogonadism and ED who have already failed PDE5 inhibitor therapy or who also have low libido. Hypogonadal patients with borderline erectile rigidity are most likely to benefit from testosterone supplementation. 1 TRT may cause increased levels of haemoglobin or haematocrit, which is associated with an increased risk of heart attack, stroke and blood clots, and can also cause an enlarged prostate or other prostate disorders. During TRT
treatment, the prostate specific antigen (PSA) will be measured to monitor for any changes and this is particularly important in men over 45 years of age. 8 As a result of using testosterone replacement, natural production of testosterone may be reduced. This may lead to a reduction in sperm production and fertility. Other side-effects of TRT include weight gain, increased appetite, hot flushes, acne, depression, restlessness, irritability, aggression, tiredness, general weakness, and excessive sweating. 8
Lifestyle modifications are considered first-line therapy for ED and men should be encouraged to make the necessary changes to benefit both their sexual function and their overall health. Recommended lifestyle modifications include increased physical activity, improved diet, stopping smoking, drugs, and alcohol, and good control of diabetes, lipids, and cholesterol. The patient's medication history should be carefully reviewed to remove or alter the doses of any medications which may be contributing to ED. Men who have a psychological cause should be offered psychosexual counselling. 1,6
PDE5 inhibitors improve erectile quality for most men and work by enhancing blood flow in the corpora cavernosa . These medications such as sildenafil are generally used on demand, and need to be taken about an hour before sexual intimacy. Tadalafil is a longer-acting daily preparation medication, therefore eliminating the ‘on-demand’ need. It is important for men to be aware that PDE5 inhibitors do not initiate the erectile response. Sexual stimulation is required to release nitric oxide from the vascular endothelium and penile nerve endings to commence the erectile process. PDE5 inhibitors are highly effective and have an overall success rate of up to 76 per cent. 1 They are contraindicated in patients taking nitrates, but otherwise are safe and
effective. When PDE5 inhibitors are coadministered with nitrates, pronounced systemic vasodilation and severe hypotension can occur.6 PDE5 inhibitors and α-adrenergic receptor blockers, often used for treatment of BPH, need to be taken at least four hours apart. 2 Among second-line therapies, external vacuum constriction devices (VCDs) are a good, nonsurgical option for patients with ED. VCDs are clear plastic chambers placed over the penis, tightened against the lower abdomen with a mechanism to create a vacuum inside the chamber. This directs blood into the penis. If an adequate erection occurs inside the chamber, the patient slips a small constriction band off the end of the VCD and onto the base of the penis. An erection beyond 30 minutes is not recommended. While cumbersome, these devices are considered safe. 6 Practice with the device improves outcomes and some degree of manual dexterity is required. VCD devices are the most inexpensive long-term therapy for ED, and a non-invasive option for patients who deem it acceptable. While the efficacy rates of VCD devices are high, patient satisfaction rates are lower. 1 Other second-line therapy includes the use of either ICI or IUS. A small needle is used to inject the ICI medication into the lateral aspect of the penis through a small-gauge needle. These vasoactive agents include prostaglandin E1, papaverine, and phentolamine and sometimes atropine, which work alone or in combination to elicit an erection. Response is dose related, usually occurs within 10-to-15 minutes, and does not require stimulation. A concern with ICI use is priapism, and if this occurs the patient will need to seek urgent medical attention. Bruising can also occur, due to it being an injected medication. The intraurethral suppository consists of a tiny pellet of prostaglandin E1 inserted into the
urethral meatus. Response is dose related, and onset usually occurs within 10-to-15 minutes. Patients need to be trained on the technique of the IUS before use, and should be advised that pain or burning may occur with this medication. 2,6
In men who fail to respond to first or second-line therapy, or who are not interested in conservative therapies, penile prosthesis implantation is available. Penile implants include malleable and inflatable devices, although most implants used are of the inflatable variety. Adverse effects including malfunction and infection are rare, and patient satisfaction is high.6
Outlook and future therapies for ED
Clinical studies in gene therapy are looking towards replacing proteins that may not be functioning properly
in the penile tissue of men with ED. Replacement of these proteins may result in improvement in ED, as has already been demonstrated in animal models given experimental gene therapy. Human studies may demonstrate success with this therapy in the future. 6
Stem cell studies may also provide advancements in the treatment of ED in the future. The mechanism of action of stem cells is to generate angiogenesis with subsequent increase in cavernosal smooth muscle cells within the corporal bodies. 9
The clinical studies published to date provide encouraging results, with improvement of sexual function reported with no side effects. Although pioneering, stem cell studies to date are small scale, with a short follow-up period, various aetiologies of ED and without a control group. 1
Melanocortin activators are drugs that act through the central nervous system, and have been shown in animal studies to produce an erection. Initial studies in humans suggest that the drug PT-141 can be effective if given intranasally in men with psychological rather than physical causes, and mild to moderate ED. Larger studies are necessary, however, to demonstrate the safety and overall effectiveness of these drugs. 9
Another potential new treatment for ED is penile low-intensity shock wave lithotripsy. This consists of 1,500 shocks twice a week for three-to-six weeks. The purpose is to stimulate neovascularisation to the corporal bodies with improvement in penile blood flow and endothelial function. The use of low-intensity shock wave lithotripsy may convert PDE5 inhibitor non-responders to responders. 1
REFERENCES
1. Leslie S, Sooriyamoorthy T. (2022). Erectile Dysfunction. StatPearls Publishing 2022. Available at: www. ncbi.nlm.nih.gov/books/NBK562253/
2. Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, Goldfarb S, et al. Erectile dysfunction. Nat Rev Dis Primers. 2016 Feb 4;2:16003. doi: 10.1038/nrdp.2016.3
3. Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients? Int J Impot Res. 2006 Jan-Feb;18(1):55-60. doi: 10.1038/sj.ijir.3901371
4. International Index of Erectile Dysfunction Questionnaire. Available at: www.urologypartners.co.uk/ repository/originals/The_International_ Index_of_Erectile_Function.pdf
5. Science Direct (2017). International Index of Erectile Function. Available at: www.sciencedirect.com/topics/ medicine-and-dentistry/internationalindex-of-erectile-function
6. Mobley DF, Khera M, Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J. 2017 Nov;93(1105):679-685. doi: 10.1136/postgradmedj-2016-134073
7. Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007 Aug 23;357(8):762-74. doi: 10.1056/ NEJMoa067423
8. NHS Kings College (2021). Testosterone replacement therapy. Information for patients. Available at: www.kch.nhs.uk/Doc/pl%20-%20 934.1%20-%20testosterone%20 replacement%20therapy.pdf
9. Khera M, Albersen, M, Mulhall J. Mesenchymal stem cell therapy for the treatment of erectile dysfunction. J Sex Med 2015; 12:1105-6. doi: 10.1111/ jsm.12871
10. Frey A, Sønksen J, Fode M. Low-intensity extracorporeal shockwave therapy in the
treatment of postprostatectomy erectile dysfunction: A pilot study. Scand J Urol 2016; 50:15. doi:10.3109/21681805.2015.1100675
11. Capogrosso P, Colicchia M, Ventimiglia E, Castagna G, Clementi MC, Suardi N, et al. One patient out of four with newly diagnosed erectile dysfunction is a young man – worrisome picture from the everyday clinical practice. J Sex Med. 2013 Jul;10(7):183341. doi: 10.1111/jsm.12179

12. Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D. Prevalence of erectile dysfunction among young adults: Results of a large-scale survey. J Sex Med 2004; 1:284–291
13. Papagiannopoulos D, Khare N, Nehra A. Evaluation of young men with organic erectile dysfunction. Asian J Androl 2015; 17:11–16
14. Najari B, Kashanian J. Erectile Dysfunction. JAMA 2016;316(17):1838. doi: 10.1001/jama.2016.12284
MELANOMA SKIN CANCER -
PREVENTION AND EARLY DIAGNOSIS
MALIGNANT MELANOMA, THE MOST SERIOUS TYPE OF SKIN CANCER, IS THE FIFTH MOST COMMONLY DIAGNOSED CANCER IN IRELAND, ACCOUNTING FOR ONE-IN-20 NEW CANCER CASES
Malignant melanoma, the most serious type of skin cancer, is the fifth most commonlydiagnosed cancer in Ireland (excluding non-melanoma skin cancer), accounting for one-in-20 new cancer cases. Every year, over 1,000 people in Ireland will be diagnosed with this disease, with males and females affected equally. The incidence of melanoma almost trebled between 1994 and 2017 in Ireland, 1 and is expected to increase by 172 per cent between 2015 and 2045, potentially giving rise to 3,078 new cases of melanoma annually. 2 Melanoma disproportionately affects younger age groups, with one-in-three cases (33 per cent) occurring in people under 50 years of age. 3
Survival rates for melanoma skin cancer are high and it accounts for just 1.8 per cent of cancer deaths in Ireland, equating to approximately 170 deaths per year. Overall, 88 per cent of people diagnosed with melanoma are still alive 10 years after diagnosis. However, early diagnosis is strongly associated with improved survival; 97 per cent of people
diagnosed with stage I melanoma are alive 10 years after diagnosis, compared to just one-in-two (48 per cent) people with stage IV disease.4
Risk factors for melanoma Ultraviolet (UV) light
The single biggest risk factor for melanoma is exposure to ultraviolet (UV) light, such as sunlight or sunbed use. It is estimated that up to 90 per cent of cases of melanoma are caused by exposure to UV light. Sunlight comprises a number of wavelengths from infrared to UV light. UV rays, specifically UVA and UVB, are damaging to skin, causing DNA damage that can result in cancer. 5
Sunburn, especially during childhood, is particularly harmful as it increases risk of melanoma later in life, but even non-burning, intermittent exposure to UV light can increase risk.
Exposure to UV light through use of sunbeds also increases risk. A recent study of 31 European Union (EU) and European Free Trade Association (EFTA) countries found that sunbed use accounted for approximately 5.1 per cent (n=4,450) of all newly-diagnosed cases of melanoma across these countries combined in 2012, with an associated economic cost of between €32.5-33 million. 6
Skin type
The effects of UV exposure on skin are not the same for everyone. A person’s natural skin colour influences their risk of UV damage and skin cancer. Most people living in Ireland have fair skin (Fitzpatrick type I and II), which burns easily and tans poorly, placing them at increased risk of UV damage and skin cancer.

Family history
About 10 per cent of patients with
THE SINGLE BIGGEST RISK FACTOR FOR MELANOMA IS EXPOSURE TO ULTRAVIOLET (UV) LIGHT
melanoma skin cancer have a family history of melanoma.7 Risk is increased in people who have a first-degree relative (parent, sibling, child) who had melanoma at a young age (younger than 30 years). Carriers of BRCA 2 gene mutations have an increased risk of melanoma.
Other risk factors
Other risk factors for melanoma include:
A new or changing mole (this is the most important sign of a possible melanoma).

Atypical moles.
A large number of moles (>50).
A previous history of melanoma or other non-melanoma skin cancer.
Immunosuppression.
ADOPTINGMelanoma prevention Avoid exposure to UV light
Up to 85 per cent of melanoma skin cancers are due to exposure to UV radiation. 8 UV rays are invisible and unlike infrared rays that cause heat, they cannot be seen or felt on our
UV radiation our skin is exposed to at any time. When the UV index is three or above, skin needs to be protected. Typically in Ireland, the UV index is three or above from April to September, even when it is cloudy. Limit time in the sun when UV rays are strongest; typically between the hours of 11:00am-3:00pm.
Wear protective clothing
Use of appropriate sun-protective clothing is one of the most important tools for sun protection. Cover skin as much as possible, wear long sleeves and collared t-shirts. To protect skin and eyes from UV radiation damage, wear a hat that protects the face, back
stipulates a UPF (ultraviolet protection factor) greater than 40. For those garments that do not have a UPF label, some items will provide better sun protection than others:
Fabric that is tightly-woven and not transparent.
Clothing that gets wet or stretched may lose some of its protective qualities.
Dark clothes block more UV rays than light-coloured clothes.
Loose-fitting clothing is better because it is less likely to become stretched and allow UV light to penetrate.
Remember to wear sunglasses with appropriate UV protection.
Use sunscreen
of the neck, eyes and ears. Wide-brim, bucket, or legionnaire hats offer the best protection from UV radiation. Baseball caps are not recommended, as these styles do not protect the ears, cheeks or neck.
The European standard for sun
The ideal sunscreen would prevent 100 per cent of UV radiation from penetrating the skin and would provide long-term protection without the need for re-application. Unfortunately, such a sunscreen does not exist. Therefore, sunscreen alone is not sufficient to protect the skin, but should be used alongside other protective measures such as clothing and shade.
Sunscreen should be applied thickly and re-applied regularly (every two hours). Remember that water-resistant sunscreen can be rubbed off by clothing or towelling.
Sunscreens should provide
BY
APPROPRIATE SKIN-PROTECTIVE BEHAVIOURS, THE MAJORITY OF SKIN CANCERS CAUSED BY HARMFUL UV RADIATION COULD BE PREVENTEDFIGURE 1: ABCDE lesion system for melanoma
(indicated by the sun protection factor (SPF) rating) and also UVA rays (indicated by a star rating). Use a broad-spectrum sunscreen with an SPF of at least 30+ for adults and 50+ for children.
By adopting appropriate skinprotective behaviours, the majority of skin cancers caused by harmful UV radiation could be prevented.
Diagnosing melanoma early
Early diagnosis of melanoma is a critical first step in achieving higher survival rates and reducing treatment severity.
It is crucial that healthcare professionals and patients can recognise the early signs of melanoma. If a GP suspects that a patient has melanoma skin cancer they can refer that patient to a pigmented lesion clinic (PLC) or to a dermatology or plastic surgery service. The National Melanoma Referral Guidelines , available here www.hse.ie/eng/ services/list/5/cancer/profinfo/ resources/gpreferrals/nationalmelanoma-gp-referral-guidelines.pdf, aim to guide decision-making around which patients require referral.
Suspicious lesions which may require urgent referral include:
Any new or changing pigmented lesion.
A long-standing pigmented lesion which is changing progressively in shape, size or colour regardless of age.
A new pigmented line in a nail, especially where there is associated damage to the nail, or a lesion growing under a nail.
A pigmented lesion which has changed in appearance or which is persistently itching or bleeding.
An ‘ugly duckling’ pigmented lesion, which is one that looks different to all the other pigmented lesions that person has.
The ABCDE lesion system ( Figure 1) can help to determine whether a mole or skin lesion is suspicious for melanoma.
Shave excisions and punch biopsies should not be carried out on naevi. If a patient presents with a suspicious pigmented lesion, the patient should be referred with the lesion intact to a consultant dermatologist or consultant plastic surgeon. If a melanoma has been inadvertently excised, the patient should be referred urgently to a consultant dermatologist or consultant plastic surgeon for multidisciplinary follow-up and care.
Resources for healthcare professionals
Currently, GPs in most regions in Ireland have access to PLCs. Patients should be referred to the PLCs using the electronic National Pigmented Lesion GP Referral Form. This form can be accessed via all of the four accredited GP computer software systems or by contacting Healthlink (www.healthlink.ie) . If your region is not covered by a PLC, you can refer a patient with suspected melanoma to your local dermatology or plastic surgery service for urgent review.
The National Cancer Control Programme10 (NCCP) has developed two early detection of cancer modules, which are available free of charge to all healthcare professionals via HSeLanD, the HSE’s national online learning and development portal. These offer a valuable learning opportunity for melanoma and other skin cancers and are available at www.hseland.ie.
Resources on skin cancer prevention are also available at www.hse.ie/sunsmart/.
A significant proportion of melanoma cases are preventable by adopting skin protective behaviours. GPs and general practice nurses (GPNs) should take every opportunity to counsel patients about sun avoidance, sun protection, and early diagnosis of melanoma. GPs and GPNs are also encouraged to

opportunistically assess patients attending their practice for signs of skin malignancy.
REFERENCES
1. National Cancer Registry Ireland (NCRI). Cancer incidence statistics. (Accessed April 2022). www.ncri.ie/ data/incidence-statistics
2. NCRI. Cancer incidence projections for Ireland 2020-2045. 2019. www.ncri.ie/publications/ cancer-trends-and-projections/ cancer-incidence-projectionsireland-2020-2045
3. NCRI. Melanoma factsheet. www.ncri.ie/sites/ncri/files/ factsheets/Factsheet%20 melanoma.pdf
4. NCRI. Survival statistics: Melanoma. www.ncri.ie/data/ survival-statistics
5. Maslin DL. Do sunscreens protect us? Int J Dermatol. 2014 Nov;53(11):1319-23. doi: 10.1111/ ijd.12606. Epub 2014 Sep 10
6. Krensel M, Schäfer I, Augustin M. Modelling first-year cost-ofillness of melanoma attributable to sunbed use in Europe. J Eur Acad Dermatol Venereol 2019 Mar;33 Suppl 2:46-56. doi: 10.1111/jdv.15313
7. Goldman L, Schafer A (eds). Melanoma and non-melanoma skin cancers. Goldman-Cecil Medicine. 26th ed. Elsevier; 2029: 1344-50.e2
8. Cancer Research UK. Risks and causes of melanoma. www. cancerresearchuk.org/aboutcancer/melanoma/risks-causes
9. Gambichler T, Laperre J, Hoffmann K. The European standard for sun-protective clothing: EN 13758. J Eur Acad Dermatol Venereol 2006 Feb;20(2):125-30. doi: 10.1111/j.1468-3083.2006.01401.x
10. Department of Health. National Cancer Strategy 20172026. 2017. www.gov.ie/en/ publication/a89819-nationalcancer-strategy-2017-2026/
TESTICULAR CANCER
TESTICULAR CANCER IS THE MOST COMMON CANCER IN MEN AGED 15-TO-45 YEARS AND REPRESENTS ONE OF THE MOST COMMON CURABLE MALIGNANCIES WHEN IDENTIFIED PROMPTLY AND TREATED WITH A MULTIMODAL APPROACH
Testicular cancer is a rare disease, representing 1 per cent of male cancers and 5 per cent of urological cancers worldwide.1 Its incidence has increased in the past few years, especially in Western and industrialised countries.
It is also the most common cancer found in young men aged between 15 and 34 years, and approximately 173 men are diagnosed with testicular cancer in Ireland each year.7
Mortality associated with testicular cancer is relatively low at approximately 0.1 per cent of all annual cancers, and cure rates are greater than 90 per cent for all stages, with over 95 per cent fiveyear survival rates.1,9

The rise of testicular cancer in Western and industrialised countries in recent years is possibly due to an increased exposure to aetiological factors. The highest incidence of testicular cancer is observed in Western and Northern Europe at 8.7 and 7.2 per 100,000 men, respectively.9
Testicular cancer includes several types of cancer, such as germ cell tumours (GCT), sex cord-gonadal stromal tumours, and secondary testicular tumours. About 90-to-95 per cent of testicular tumours arise from germ cells to generate the GCT, followed by 5-to-10 per cent gonadal stromal tumours, mixed GCT and secondary tumours. 2
Anatomy and physiology of the testes
The testes (male gonads) are located in a skin-covered, highly pigmented, muscular sack called the scrotum that extends from the body behind the penis. They produce both sperm and androgens such as testosterone and are active throughout the reproductive lifespan of the male. Paired ovals, the testes are approximately 4-to-5cm in length and are surrounded by two distinct layers of protective connective tissue. The outer tunica vaginalis is a serous membrane that has both a parietal and a thin visceral layer. Beneath the tunica vaginalis is the tunica albuginea, a tough, white, dense
connective tissue layer covering the testis, which also inverts to form septa that divide the testis into 300-to-400 structures called lobules. Within the lobules, sperm develop in structures called seminiferous tubules. 8
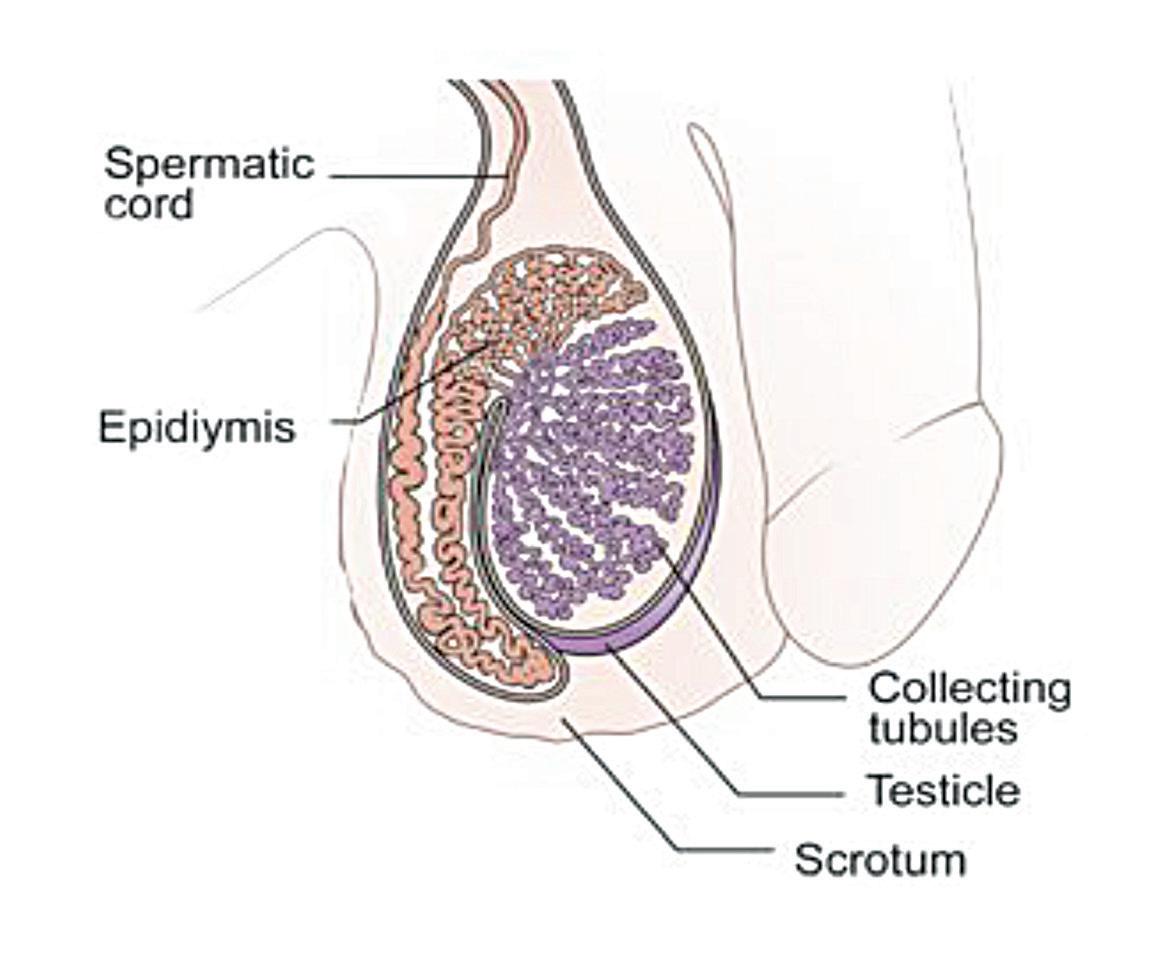
Risk factors
The main non-modifiable risk factors for testicular cancer are cryptorchidism (undescended testicle), family history, previous history of testicular cancer, genetic predisposition, ethnicity, congenital abnormalities, and infertility. 2
In cryptorchidism, the undescended testicle remains in the abdomen or groin, and the risk of developing the disease
does not change even after surgery to move the testicle into the scrotum. In patients with cryptorchidism, the relative risk of developing testicular cancer ranges from 2.9-to-6.3. The risk is increased in both testes, although the risk is much higher in the ipsilateral testis (6.3 vs 1.7).5 Family history correlates to an increased risk and testicular cancer risk is significant in men whose father or brother had the disease. Patients with a father or brother with testicular cancer have a 3.8- and 8.6-times greater risk, respectively.5
Those diagnosed with cancer in one testicle are also more likely to get cancer in the other testicle. Patients with a personal history of testicular cancer have a 12-times greater risk of developing a contralateral testicular cancer than the general population. However, the greatest risk is in the first five years after diagnosis, and the 15-year cumulative risk is 1.9 per cent.5
Genetic and environmental factors play an important role in the genesis and development of testicular cancer. Several genes are implicated in its pathogenesis and different environmental factors have been investigated.1 Klinefelter’s syndrome,
caused by a chromosomal abnormality, has been associated with testicular cancer and other cancer types, and congenital abnormalities of the testicles, penis or kidneys may also contribute to an increased risk of testicular cancer.
Age represents one of the most frequent factors of testicular cancer occurrence and the highest incidence of GCT has been found in men between 15 and 35 years of age.
Infertility is also strongly associated with testicular cancer.
Caucasian men have a higher chance of getting the disease than Afro-Caribbean or Asian men.1,3
Symptoms
Testicular cancer may present as a painless scrotal mass, an incidental radiologic finding, post-traumatic symptom, or scrotal pain. 5 An enlarged testicle or a small lump or area of hardness are usually the first signs of testicular cancer. Any lump, enlargement, hardness, pain, or tenderness in the testicle should be evaluated as soon as possible. Other symptoms of testicular cancer usually do not appear until after the cancer has spread to other parts of the body.
Symptoms of testicular cancer may include:4
A painless lump or swelling on either testicle. Found early, a testicular tumour may be about the size of a pea or a marble, but it can grow much larger.
Pain, discomfort, or numbness in a testicle or the scrotum, with or without swelling.
Change in the way a testicle feels or a feeling of heaviness in the scrotum. For example, one testicle may become firmer than the other or testicular cancer may cause the testicle to grow bigger or to become smaller.
Dull ache in the lower abdomen or groin.
Sudden build-up of fluid in the scrotum.

Although rare, some testicular tumours make hormones that cause gynaecomastia.
Lower back pain, shortness of breath, chest pain, and bloody sputum or phlegm can be symptoms of laterstage testicular cancer.
Swelling of one or both legs or shortness of breath from a blood clot can be symptoms of testicular cancer. When cancer spreads to other sites such as the lungs, brain, abdomen, or neck, symptoms including nausea, vomiting, gastric upset, cough, shortness of breath, weakness, sensory disturbances, abdominal pain, lumps in the neck/groin areas, and back pain can occur. Prompt evaluation is important
REMOVING ONE TESTICLE DOES NOT AFFECT LIBIDO OR THE ABILITY TO HAVE AN ERECTION PROVIDING THE REMAINING TESTICLE IS NORMAL
to ensure early diagnosis and treatment and decrease the burden of treatment in advanced disease. 9
Testicular cancers are defined based on their cell type. The most common histology of testicular cancer is germinal-seminoma and non-seminoma. About 95 per cent of testicular cancers begin in germ cells, specialised cells in the testicles that make sperm. While these tumours typically start in the testicles they also occasionally arise in the abdomen, chest, or other areas of the body, even if there is no evidence of cancer in or near the testicles. Seminomas make up about half of all GCTs. They usually grow slowly and are less likely to metastasise to other parts of the body. Non-seminomas are often more aggressive than seminomas, and more likely to spread beyond the testicle. Approximately 5 per cent of testicular cancers start in stromal cells, which make testosterone. Testicular stromal tumours are often benign. 3
Diagnosis
Evaluation by clinicians is guided by a complete history of the presenting symptoms, physical examination, and assessment for risk factors. Testicular examination should include the affected and unaffected testis for comparison. The normal testis is 3.5-5cm in length, smooth, homogenous, movable, and detached from the epididymis. Hard, firm, or fixed areas within or adjacent to the testes are abnormal and warrant further investigation. Physical examination should also include evaluation of the inguinal and supraclavicular lymph nodes, the abdomen, and the chest for gynaecomastia. 5 It is important to ask specifically about the history of cryptorchidism, orchiopexy, or inguinal hernia repair as an infant. A family history of testicular cancer in the father or a brother should be elicited. Physical examination findings of any solid intra-testicular mass should be considered to be testicular cancer until
proven otherwise. 9
Many signs and symptoms of testicular cancer are similar to those caused by non-cancerous conditions such as a spermatocele, varicocele, hydrocele, inguinal hernia, lymphoma, epididymo-orchitis or epididymitis, and differential diagnosis is important.4
Blood tumour markers include Alpha-fetoprotein (AFP), beta human chorionic gonadotrophin (bHCG), and Lactate dehydrogenase (LDH).6
Scrotal ultrasonography can confirm the presence of a mass, and has a sensitivity of 92-to-98 per cent and specificity of 95-to-99.8 per cent.5
active surveillance, chemotherapy, retroperitoneal lymph node dissection, and radiation therapy. 9
Stages are based on four categories:11,12
T (Tumour): This describes whether the tumour has spread to tissues near the testicle.
N (Node): Indicates whether the testicular cancer cells have spread to regional lymph nodes.
M (Metastasis): This refers to whether the cancer has metastasised.
S (Serum): This indicates the level of tumour marker proteins in the serum, or blood.
Once the individual T, N, M, and S components are scored, they are combined to determine the overall testicular cancer stage group.
The stages of testicular cancer are: Stage 0: The cancer cells have not spread beyond the testicle. At this stage, tumours are also referred to as carcinomas in situ.
Stage 1 testicular cancer: The cancer has invaded tissues next to the testicle, but has not spread to lymph nodes, or more distant sites in the body. Levels of tumour marker proteins may be normal or elevated. The three subcategories of stage 1 testicular cancer are:
Once a solid intra-testicular tumour is identified, radical inguinal orchiectomy is performed both for diagnostic and therapeutic purposes.12 A biopsy of the suspect mass will be carried out and when suspicion for metastatic disease is present, additional imaging with CT of the chest and abdomen may be done for staging. 5
Staging
Staging is determined by the size of the tumour, lymph node involvement, whether the cancer has spread and if tumour markers are present.10
Tumour staging guides further management with options including
Stage 1A: The tumour may have grown through the inner layer of tissue surrounding the testicle, but not the outer layer, and it has not spread to blood or lymph vessels. Serum levels of tumour markers are normal.
Stage 1B: Tumours at this stage may have spread to blood or lymph vessels or may have invaded the outer layer surrounding the testicle, the spermatic cord or the scrotum. Serum levels of tumour markers are normal.
Stage 1C: These cancers can demonstrate any degree of invasion of nearby tissues, and levels of tumour markers measured after the tumour has been removed by surgery are elevated.
SINCE TREATMENT IS SUCCESSFUL FOR MOST MEN WITH TESTICULAR CANCER, ONE OF THE MAJOR FUTURE GOALS IS TO REDUCE THE SIDEEFFECTS OF TREATMENT FOR THOSE WITH EARLY-STAGE CANCER
Stage 2 testicular cancer: Testicular cancers at this stage have invaded tissues next to the testicle and can now be found in at least one nearby lymph node. Tumour marker levels may be normal or slightly elevated. Stage 2 testicular cancer has three subcategories:
Stage 2A: Tumours at this stage have spread to one or more lymph nodes, but no node is larger than 2cm.
Stage 2B: Tumours at this stage have spread to at least one lymph node, which is between 2-5cm in size.
Stage 2C: These tumours have spread to at least one lymph node larger than 5cm.
Stage 3 testicular cancer: Testicular cancers at this stage have spread to distant lymph nodes or organs. Stage 3 testicular cancer has three subcategories:
Stage 3A: These cancers have spread to a distant lymph node or the lungs. Tumour marker protein levels are normal or slightly elevated.
Stage 3B: At this stage of testicular cancer, patients have moderately elevated levels of tumour marker proteins, and the disease has either spread to nearby or distant lymph nodes or the lungs.
Stage 3C: These cancers have high levels of tumour marker proteins and may have spread to nearby or distant lymph nodes, or the lungs. Alternatively, they may have spread to other distant organs such as the liver or the brain, but in this case serum tumour markers can be at any level.
Treatment and prognosis
Treatment options for testicular cancer include surgery, radiation therapy, chemotherapy and stem cell transplant. Sometimes more than one type of treatment might be used including chemotherapy and/or radiotherapy. 9
The management of seminomas depends on the extent of spread of the cancer. Surgery, radiotherapy
and chemotherapy are used to treat seminomas and the stage of cancer will decide treatment options. Nonseminomas are usually treated with surgery and chemotherapy.10
When a diagnosis of testicular cancer is suspected based on physical examination and ultrasound findings, radical inguinal orchiectomy is performed, which removes the testicle, epididymis, and spermatic cord up to the level of the internal inguinal ring. In this procedure, these structures are delivered through an inguinal incision made along Langer’s lines in the groin. If the mass is too large to pass through a standard 3-to-5cm inguinal incision, the incision can be carried inferiorly to the anterior scrotum to allow for removal of the testis in its tunics along with the spermatic cord. Trans-scrotal orchiectomy or biopsy is contraindicated, as doing so alters the lymphatic drainage patterns and impacts further management. Further surgery or radiotherapy or chemotherapy will be based on the disease's stage and response to the initial management.9
Following radical inguinal orchiectomy, patients should avoid heavy lifting and high-impact activities for four weeks and should wear supportive underwear to prevent scrotal swelling or haematoma. Retroperitoneal lymph node dissection is major intra-abdominal surgery, after which dedicated post-operative rehabilitation should be undertaken. 9
Removing one testicle does not affect libido or the ability to have an erection providing the remaining testicle is normal. The removal of a testicle may be traumatic, especially for a young man, and a testicular prosthesis can be placed in the scrotum at the time of surgery. Fertility can be compromised by testicular cancer treatment. However, the potential to father children should not be greatly affected provided the other testicle is normal. Chemotherapy, however, does affect sperm production in this testicle
and it is recommended that patients with testicular cancer arrange to freeze sperm in case there are problems with fertility later on.7 Adequate counselling should be given regarding the possibility of infertility, sperm-banking and also regarding the placement of a testicular prosthesis if required.9
Prognosis is determined by the histology, extent of distant tumour spread, and extent of tumour marker elevations. For men with disseminated seminomas, the main adverse prognostic variable is the presence of metastases to visceral organs other than the lungs. A tumour that originated in the mediastinum has a worse prognosis when compared to a tumour that originated within the testicle.9
Patient education and testicular self-examination
There is no national screening programme for detecting testicular cancer in Ireland.6 Testicular selfexamination is one of the simplest and most effective ways to identify testicular cancer early, although there is controversy about its efficacy.7 Despite opportunities for an early diagnosis, most men seek medical help only after some time has passed, when symptoms have intensified, or after receiving information about the condition from someone else or the media. Despite the fact that knowledge about testicular cancer and testicular self-examination in developed counties is higher, testicular self-examination is still rarely performed. Most patients present with a testicular mass, which suggests that testicular selfexamination could aid early detection.1 It is important to educate men of all ages about testicular cancer and testicular self-examination and that healthcare professionals, especially nurses, are well informed and able to discuss it with their patients. Healthcare professionals play a key role in providing information about testicular cancer
risk factors and symptoms and in explaining the importance of testicular self-examination.7 Mortality associated with testicular cancer is relatively low at approximately 0.1 per cent of all annual cancer death.
Testicular selfexamination
Often the best place to check is in the bath or shower where the scrotum is relaxed and the testicles can be felt easily.
Hold the scrotum in both hands.
Use your fingers and a thumb to examine the testicles.
It is common for one testicle to be slightly larger than the other.
Gently feel each testicle, one at a time.
You should be able to feel a soft tube at the top and back of both testicles. This tube called the epididymis carries the sperm. It may be slightly tender, but do not confuse this with an abnormal lump in the testicles.
If you notice a lump or anything
unusual contact your GP immediately. The GP will be able to assess and if necessary, refer to a consultant for further investigations.
Do not be embarrassed or nervous.
Remember early detection of the disease is the best chance of a cure.
Testicular self-examination should be performed every month.7 Men tend to seek help late for testicular problems for many reasons such as anxiety and fear of receiving an undesired diagnosis. Others feel ashamed, deprived of their masculinity and are too embarrassed to talk about it with anyone even a partner. Researchers have found that when men are properly educated about testicular self-examination they are more likely to carry it out and recognise symptoms of testicular cancer.1

Outlook
Testicular cancer is the most common cancer in men aged 15-to-45 years and represents one of the most common curable malignancies when identified
promptly and treated with a multimodal approach. 9 It has excellent survival rates and having awareness about the disease and seeking prompt healthcare attention is of utmost importance. Since treatment is successful for most men with testicular cancer, one of the major future goals is to reduce the side-effects of treatment for those with early-stage cancer. In addition, treatments for poor-risk and recurrent cancers are being studied in clinical trials, along with research on the causes and genetics of testicular cancer. Stem cell transplant is most often used to treat testicular cancers that have re-occurred after treatment with chemotherapy. Current studies are looking at whether a stem cell transplant may be valuable as part of the first treatment for some patients with advanced germ cell cancers. Clinical trials are also underway to find better ways of reducing symptoms and side-effects of current testicular cancer treatments, so as to improve patients' comfort and quality-of-life.13
REFERENCES
1. Bresciani M, Boarin M, Facconi L, Manara F, Villa G. Awareness of testicular cancer among young men: A literature review. Int J Urol Nurs 2020 Jul 17;15(1): 5-11. doi: 10.1111/ ijun.12248
2. Boccellino M, Vanacore D, Zappavigna S, Cavaliere C, Rossetti S, D'Aniello C, et al. Testicular cancer from diagnosis to epigenetic factors. Oncotarget. 2017 Sep 18;8(61):104654-104663. doi: 10.18632/ oncotarget.20992
3. MSK (2021). Testicular cancer: Germ cell tumours. Available at: www.mskcc. org/cancer-care/types/testicular-germcell-tumors
4. Cancer.Net (2020). Testicular cancer: Symptoms and signs. Available at: www. cancer.net/cancer-types/testicularcancer/symptoms-and-signs
5. Baird D, Myers, G, Darnall C, Hu J. Testicular cancer: Diagnosis and treatment. Am Fam Physician 2018 Feb 15; 97(4):261-268. www.aafp.org/afp/2018/0215/p261. html
6. Irish Cancer Society (2021). Symptoms and diagnosis of testicular cancer. Available at: www.cancer.ie/ cancer-information-and-support/ cancer-types/testicular-cancer/ symptoms-and-diagnosis-of-testicularcancer
7. Marie Keating Foundation (2021). Testicular cancer. Available at: www. mariekeating.ie/cancer-information/ testicular-cancer/
8. Anatomy and physiology of the male reproductive system. Available at: https://courses. lumenlearning.com/suny-ap2/ chapter/anatomy-and-physiology-ofthe-male-reproductive-system/
9. Gaddam, S. (2021). Testicular cancer. StatPearls: Available at: www.statpearls. com/ArticleLibrary/viewarticle/29984
10. St James’s Hospital (2021). Testicular cancer. Available at: www.stjames.ie/ cancer/typesofcancer/testicularcancer/
11. MacMillan Cancer support (2021). Stages of testicular cancer. Available at: www.macmillan.org.uk/cancerinformation-and-support/testicularcancer/stages
12. Markman, M. (2021). Testicular cancer stages. Available at: www. cancercenter.com/cancer-types/ testicular-cancer/stages
13. CancerNet (2020). Testicular cancer: Latest research. American Society of Clinical Oncology: ASCO. Available at: www.cancer.net/cancer-types/testicularcancer/latest-research
JUST WHAT IS THE HEALTH IMPACT OF GUT FLORA?
Iused to be very yeasty. Years with undiagnosed haemochromatosis had many effects, but one was a tendency towards sinusitis, something that vanished as soon as I was thoroughly de-ironed. But I suspect all the antibiotics and even, on occasion, steroids that I had ingested during that time had left their mark on my gut microbiota. I took probiotic supplements and even took a daily dose of fructo-oligosaccharides (FOs), but it didn’t seem to make much difference. I just didn’t feel great. It was when I went low carb – keto initially – that everything seemed to improve. I was no longer feeding the nasty micro-organisms with their favourite food, sugar.
I’ve stuck with the low carb regime most of the time ever since. But I’ve just had a birthday weekend that involved some quite wonderful baking and I have to say I can sense the difference. It would be indelicate to be too specific, but let’s just say that it’s a gaseous issue. Back to the normal routine now.
Some of the world-leading research into the gut flora and its impact on multiple aspects of health including, fascinatingly, our state of mind, is carried on at University College Cork. But it was a paper from elsewhere that caught my eye lately: ‘The potential of the gut microbiome to reshape the cancer therapy paradigm’ (JAMA Oncology, 2022; doi: 10.1001/ jamaoncol.2022.0494).
The authors, from the Brigham and Women’s Hospital in Boston, one of Harvard Medical School’s teaching institutions, say that exploration of the influence of the microbiome in cancer therapy is currently being undertaken in several centres, which include trials of faecal transplants,
supplements, and new drugs that may influence microbiota make-up.
Although they refer to recent research that reports some benefits of ketogenic diets for cancer patients (including the role of beta-hydroxybutyrate or BHB in colon cancer as reported in Nature in April), they say that caution must be exercised before embracing probiotics or major dietary changes.
This caveat is significant because the issue of diet and cancer is undoubtedly extremely complex and, more to the point,
or also contained other bacteria some of which could be harmful (doi: 10.1016/j. lwt.2021.113055). However, none of the products were named. There was a recent report on omega-3 fish oil supplements that found many brands were actually rancid, meaning that they could push LDL cholesterol levels higher.
Some evidence has emerged that suggests vegan diets could help people with a BMI of 25+ to lose weight. When I mentioned this to a vegetarian friend he snorted derisively and suggested that this might be because they could find so little to eat. However, it seems that such a diet can lower blood sugar in people with type 2 diabetes. Although the trials from which the data was drawn involved almost 800 people, I was surprised to see that there was no mention of what kind of vegan diet was embraced by the participants. 'Vegan' is surely a broadish church?
emotive. The irritatingly cheerful twin vegetarians who go by the name of The Happy Pear got into very hot water – and deservedly so – for saying, essentially, that women with breast cancer have probably not eaten a “healthy” diet, whatever that may be. They have apologised for their tone-deaf comments.
I know from experience that probiotics vary enormously in their potency, and it would be good to see a consumerfriendly league table-based independent analysis. There was an interesting paper from Russian researchers in February that examined probiotics from all over Europe and found that many did not contain what was claimed on the label

Eating nothing but, say, sourdough bread spread with Marmite would be a vegan diet, and quite tasty, but it wouldn’t be very good for anyone in isolation. Healthy vegans have to take a very detailed view of their eating habits in order to thrive. Omnivores don’t have that problem and are, perhaps, more likely to make thoughtless choices concerning diet.
The world seems to be becoming more and more judgmental and, in an echo of The Happy Pear’s solecism, I often get the impression that there’s a culture of patientblaming. I know that many sick people feel guilty at having 'allowed' themselves to get unwell, as they see it. There seems to be a kind of equation of health with goodness and disease with badness and it’s something that must surely do a great deal of harm. It needs to be rooted out.
EATING NOTHING BUT, SAY, SOURDOUGH BREAD SPREAD WITH MARMITE WOULD BE A VEGAN DIET, AND QUITE TASTY ...
ACROSS
Across
1 - Surrender (6)
1 Surrender (6)
4 Walk aimlessly (6)
4 - Walk aimlessly (6)
9 Eg, biology (7)
DOWN
1 Female sibling (6)
2 Edifice (8)
3 Opposite of outer (5)
Down
1 - Female sibling (6)
SCRIBBLE BOX
2 - Edifice (8)
9 - Eg biology (7)
10 Extraordinary occurrence (7)
11 Senior figure in a tribe (5)
12 Pertaining to the voice (5)
5 Sanction (7)
6 Circular storage medium (4)
10 - Extraordinary occurrence (7)
14 Sense experience (5)
15 Sweetening substance (5)
7 Divulge; tell (6)
8 Show (11)
11 - Senior figure in a tribe (5)
17 Cluster (5)
18 Prescription (7)
13 Coldly detached (8)
14 Victory (7)
12 - Pertaining to the voice (5)
20 Cigarette constituent (7)
21 Sense of musical time (6)
15 Experience pain (6)
16 Speculative view (6)
14 - Sense experience (5)
22 Bring into action (6)
17 Religious book (5)
19 Depend on (4)
15 - Sweetening substance (5)
17 - Cluster (5)
18 - Prescription (7)
20 - Cigarette constituent (7)
3 - Opposite of outer (5)
5 - Sanction (7)
6 - Circular storage medium (4)
7 - Divulge; tell (6)
8 - Show (11)
13 - Coldly detached (8)
14 - Victory (7)
15 - Experience pain (6)
16 - Speculative view (6)
FOR ALL THAT MATTERS IN MEDICINE



SOME THINGS ARE JUST PART OF GROWING UP
WITH VARIVAX [VARICELLA VACCINE (LIVE*)], CHICKENPOX DOESN’T ALWAYS HAVE TO BE ONE OF THEM.
A FLEXIBLE APPROACH TO ADMINISTRATION1 VARIVAX can be administered intramuscularly or subcutaneously†
VARIVAX IS NOW AVAILABLE TO ORDER PRIVATELY FROM MSD.
VARIVAX® powder and solvent for suspension for injection in a pre-filled syringe [Varicella Vaccine (live)] ABRIDGED PRODUCT INFORMATION Refer to Summary of Product Characteristics before prescribing. PRESENTATION Vial containing a lyophilised preparation of live attenuated varicella virus (Oka/Merck strain) and a prefilled syringe containing Water for Injections. After reconstitution, one dose (0.5 mL) contains no less than 1350 PFU (Plaque-forming units) varicella virus (Oka/Merck strain). INDICATIONS
VARIVAX is indicated for vaccination against varicella in individuals from 12 months of age. VARIVAX can be administered to infants from 9 months of age under special circumstances, such as to conform with national vaccination schedules or in outbreak situations. VARIVAX may also be administered to susceptible individuals who have been exposed to varicella. Vaccination within 3 days of exposure may prevent a clinically apparent infection or modify the course of the infection. Limited data indicate that vaccination up to 5 days after exposure may modify the course of the infection. The use of VARIVAX should be based on official recommendations. DOSAGE AND ADMINISTRATION The vaccine should be injected intramuscularly or subcutaneously. The vaccine should be administered subcutaneously in patients with thrombocytopenia or any coagulation disorder. Do not inject intravascularly. Individuals from 9 to 12 months of age should receive 2 doses, a minimum of 3 months apart. Individuals from 12 months to 12 years of age should receive 2 doses, a minimum of 1 month apart. Individuals 12 months to 12 years of age with asymptomatic HIV infection [CDC Class 1] with an agespecific CD4+ T-lymphocyte percentage ≥25% should receive two doses given 12 weeks apart. Individuals from 13 years of age and older should receive 2 doses, given 4-8 weeks apart. If the interval between doses exceeds 8 weeks, the second dose should be given as soon as possible. CONTRAINDICATIONS Hypersensitivity to any varicella vaccine, to any of the excipients or neomycin. Blood dyscrasias, leukaemia, lymphomas of any type, or other malignant neoplasms affecting the hemic or lymphatic systems. Individuals receiving immunosuppressive therapy. Severe humoral or cellular immunodeficiency. Individuals with a family history of congenital or hereditary immunodeficiency unless immune competence has been demonstrated. Active untreated tuberculosis. Any illness with fever >38.5ºC. Pregnancy. Furthermore, pregnancy should be avoided for 1 month following vaccination. PRECAUTIONS AND WARNINGS In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Appropriate medical treatment and supervision should always be available in the rare event of anaphylaxis. Vaccine recipients should avoid salicylates for 6 weeks after vaccination. Vaccination may be considered in patients with selected immune deficiencies where the benefits outweigh the risks. Immunocompromised patients who have no contraindication for this vaccination may not respond as well as immunocompetent subjects; therefore, some of these patients may acquire varicella in case of contact, despite appropriate vaccine administration. These patients should be monitored carefully for signs of varicella. Transmission of varicella vaccine virus (Oka/Merck strain) resulting in varicella infection including disseminated disease may rarely occur between vaccine recipients (who develop or do not develop a varicella-like rash) and contacts susceptible to varicella including healthy as well as high-risk individuals. Vaccine recipients should therefore avoid close association with susceptible high-risk individuals for up to 6 weeks after vaccination. If varicella vaccine (live) (Oka/ Merck strain) is not given concomitantly with measles, mumps, and rubella virus vaccine live, a 1-month interval between the 2 live virus vaccines should be observed. Sodium: This medicinal product contains less than 1 mmol (23 mg) sodium per dose and is considered to be essentially ‘sodium-free’. Potassium: This medicinal product contains less than 1 mmol (39 mg) potassium per dose and is considered to be essentially ‘potassium-free’. PREGNANCY AND LACTATION Pregnant women should not be vaccinated with VARIVAX. Studies have not been conducted with VARIVAX in pregnant women. However, foetal damage has not been documented when varicella vaccines have been given to pregnant women. It is not known whether VARIVAX can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. Pregnancy should be avoided for 1 month following vaccination. Women who intend to become pregnant should be advised to delay. VARIVAX
not generally recommended for breastfeeding mothers.
EFFECTS Healthy individuals 12 months to 12 years of age (1 dose): Very common side effects: Fever. Common side effects: Upper respiratory infection, rash, measles /rubella-like

rash, varicella-like rash (generalised median 5 lesions), injection site erythema, rash, pain/tenderness/soreness, swelling and varicellalike rash (injection site median 2 lesions), irritability. Healthy individuals 12 months to 12 years of age (2 doses received ≥ 3 months apart): The following serious side effects temporally associated with the vaccination were reported in individuals 12 months to 12 years of age given varicella vaccine (live) (Oka/Merck strain): Diarrhoea, febrile seizure, fever, post-infectious arthritis, vomiting. Healthy individuals 13 years of age and older (majority received 2 doses 4 to 8 weeks apart): Very common side effects: Fever ≥ 37.7°C oral, injection-site erythema, soreness and swelling. Common side effects: varicella-like rash (generalised median 5 lesions), injection-site rash, pruritus and varicella-like rash (injection site median 2 lesions). Other reported adverse events (during post-marketing surveillance) that may potentially be serious include thrombocytopenia, pneumonia, encephalitis, anaphylaxis, cerebrovascular accident, febrile and non-febrile convulsions, Guillain-Barré syndrome, transverse myelitis, ataxia, Stevens-Johnson syndrome, erythema multiforme Henoch-Schönlein purpura and herpes zoster. Varicella (vaccine strain) has been reported during marketed use of the vaccine. The vaccine virus may rarely be transmitted to contacts of vaccinees who develop or do not develop a varicella-like rash. Complications of varicella from vaccine strain, including herpes zoster and disseminated disease such as aseptic meningitis and encephalitis, have been reported in immunocompromised or immunocompetent individuals. Necrotizing retinitis has been reported post-marketing in immunocompromised individuals. For a complete list of undesirable effects please refer to the Summary of Product Characteristics.
PACKAGE QUANTITIES Single vial of vaccine and prefilled syringe of diluent with two unattached needles. Legal category: POM
Marketing authorisation number: PA 1286/057/001 Marketing Authorisation holder: Merck Sharp & Dohme Ireland (Human Health) Limited, Red Oak North, South County Business Park, Leopardstown, Dublin 18, Ireland. Date of revision: July 2020. © Merck Sharp & Dohme B.V. 2020. All rights reserved. Further information is available on request from: MSD, Red Oak North, South County Business Park, Leopardstown, Dublin 18 D18 X5K7 or from www.medicines.ie. II106G (CRN009LPO)

Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie Adverse events should also be reported to MSD (Tel: 01-299 8700) WS098 (CRN008R8M)
* VARIVAX is a live attenuated vaccine contraindicated in certain patients. See prescribing information.1
† VARIVAX should be administered subcutaneously in patients with thrombocytopenia or any coagulation disorder.1
Reference 1. VARIVAX Summary of Product Characteristics February 2022.
Red Oak North, South County Business Park, Leopardstown, Dublin D18 X5K7 Ireland
