ENDOCRINOLOGY AND DIABETOLOGY



SPECIAL FOCUS ON OBESITY



Addressing stigma, new international guidelines, and the latest evidencebased treatment approaches


Full coverage of EASD 2020


































CLINICAL ARTICLES ON osteoporosis, PCOS, thyroid disorders, and diabetic retinopathy





Type 1 and 2
DIABETES






The latest drug therapies, monitoring technologies, and HSE management in primary care










VOL 6 ISSUE 8 2020

Surviving and thriving during Covid-19
Welcome to the latest edition of Update Endocrinology and Diabetology.
As health services continue to grapple with providing care in the ‘new normal’ caused by the Covid-19 pandemic, there is rising concern about missed opportunities and early warning signs being ignored in people with chronic diseases such as diabetes.
After the initial wave and early lockdown, hospital and community diabetes services began gradually resuming and scaling up delivery of care and reaching out to patients in need of check-ups and intervention, but the second wave has quickly brought fresh challenges and patients understandably remain reluctant to seek help for what might seem like minor issues.
Commenting on this worrying development recently, Dr Anna Clarke, Diabetes Ireland, said: “We know people are delaying contacting GPs and hospital diabetes teams in the belief that they are helping those professionals cope with the current Covid-19 burden, but in doing so may be risking their own health as their problem escalates. It is vital to seek medical attention early and be treated and reassured.”

In August the HSE and Diabetes Ireland launched an awareness campaign urging people with concerns about their diabetes to seek medical advice from their pharmacist, GP or hospital diabetes team and reassured them that diabetes services had resumed and are continuing despite the challenges of providing care while dealing with Covid-19.
In an interview in this issue of Update, Prof Sean Dinneen, Consultant Endocrinologist and Clinical Lead, HSE National Clinical Programme for Diabetes, highlights the importance of patients continuing to seek help despite the current challenges: “We are in a very fluid environment and diabetes services are experiencing significant capacity issues at present as the health service deals with this pandemic. However, it must be stressed that delays in seeking medical attention often results in additional care being needed.”
As he points out: “The Covid-19 pandemic presented our health service with a set of unprecedented circumstances resulting in disruption to the delivery of non-Covid care, including routine diabetes care. The remarkable work carried out by healthcare teams across the country and the support, co-operation, and understanding of patients, service users and families during the recent first phase of the Covid-19 response is very much appreciated by all in the health service.”
Throughout the pandemic, diabetes and endocrinology healthcare providers have also worked hard to adapt to remotely support and empower people in selfmanaging their conditions at home. Maintaining this self-management will continue to improve quality-of-life and reduce the impact on health and the likelihood of complications, and telehealth solutions are being supported by the HSE to continue and evolve.
Meanwhile, the Irish Society for Clinical Nutrition and Metabolism (IrSPEN) has also cautioned that patients with obesity, who are at higher risk of complications from Covid-19, need equal access to treatments during the pandemic and must not have their surgeries and other treatments indefinitely delayed.
To help manage this challenge, obesity consultants from around the world,
including Ireland, have together published guidance on prioritising access to surgery and treatments for those with obesity and associated diabetes, the details of which are featured on page 11.
This issue has a special focus on obesity, looking at the latest knowledge and addressing stigma, with expert articles on evidencebased treatment approaches including drug therapies, surgery, and specialist diets.
There are also a number of in-depth clinical articles on managing type 1 and type 2 diabetes, including the latest drug therapies, disease-monitoring technologies, closed loop insulin delivery, and an overview of the HSE’s chronic disease management programme for diabetes in primary care.
In addition, there is a comprehensive overview of diabetes and the kidney and how Covid-19 has impacted these patients, a very interesting case study article on a rare form of diabetic retinopathy from Diabetic RetinaScreen, as well as practical expert overviews of common thyroid disorders and osteoporosis, and management approaches for PCOS.
Conference wise, there is a round-up of the most topical research presented at the recent 2020 European Association for the Study of Diabetes (EASD) meeting, held virtually for the first time this year.
So all in all, a packed edition that should hopefully prove interesting and useful to all our readers.
Thank you to our expert contributors for taking the time to share their knowledge and advice for the betterment of patient care.
We always welcome new contributors and suggestions for future content, as well as any feedback on our content to-date. Please contact me at priscilla@mindo.ie if you wish to comment or contribute an article. ■
1 Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology
A message from Priscilla Lynch, Editor
24 Changing the landscape for community-based type 2 diabetes care in Ireland
Osteoporosis: The silent
32 Diabetes and the kidney in 2020; glimmers of hope in the face
Editor Priscilla Lynch priscilla@mindo.ie
Sub-editor Emer Keogh emer@greenx.ie
Creative Director Laura Kenny laura@greenx.ie
Advertisements Graham Cooke graham@greenx.ie
Administration Daiva Maciunaite daiva@greenx.ie
Update is published by GreenCross Publishing Ltd, Top Floor, 111 Rathmines Road Lower, Dublin 6 Tel +353 (0)1 441 0024 greencrosspublishing.ie
© Copyright GreenCross Publishing Ltd 2020
The contents of Update are protected by copyright. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means – electronic, mechanical or photocopy recording or otherwise – whole or in part, in any form whatsoever for advertising or promotional purposes without the prior written permission of the editor or publisher.
Disclaimer
The views expressed in Update are not necessarily those of the publishers, editor or editorial advisory board. While the publishers, editor and editorial advisory board have taken every care with regard to accuracy of editorial and advertisement contributions, they cannot be held responsible for any errors or omissions contained.
GreenCross Publishing is owned by Graham Cooke graham@greenx.ie
Contents 2 03
Study of Diabetes (EASD) 2020 Meeting report 06 Irish diabetes research: Insulin pump therapy uptake low in Ireland 08 Interview with Prof Sean Dineen, HSE Clinical Lead for Diabetes 10 Is obesity really a disease? 12 Advances in diabetes technology 14 Closed loop insulin delivery in type 1 diabetes mellitus 18 The latest treatment approaches in diabetes 21 Diabetes and the new HSE chronic disease management programme
European Association for the
26
36
2 diabetes and obesity: How to treat a twin pandemic 40 Obesity in adults – a new clinical practice guideline from Obesity Canada 44 Common thyroid disorders: An overview 49 Managing polycystic ovary syndrome 51 Diabetic retinopathy: An unusual case report
disease
of a global pandemic
Treating type
AUTHOR: Priscilla Lynch provides a round-up of some of the most topical research presented at this year’s Annual Meeting of the European Association for the Study of Diabetes (EASD), held online this year from 21-25 September 2020
A new modelling study presented at the 2020 Annual Meeting of the European Association for the Study of Diabetes (EASD) suggests that the average person with type one diabetes (T1DM) will live almost eight years less than the average person in the general population without diabetes, while those with type 2 diabetes (T2DM) will live almost two years less.

For their analysis, the authors used mortality data from the UK National Diabetes Audit (NDA) for 2015-16, and the O ce for National Statistics (ONS) published for 2015-17. The researchers took data from 6,165 general practices supporting 41.3 million people of whom 217,000 were on T1DM register and 2.50 million on T2DM register.
In their model, the authors applied relative NDA mortality rates to population rates for each age/sex, and then calculated the future life expectancy for T1DM/T2DM/ non-DM populations. The di erence between total life expectancy for the total reported populations by age and gender of T1DM and T2DM and an equivalent
population with non-DM gave the total ‘lost life years’ (LLY).
In the model the ‘average’ person with T1DM (age 42.8 years) has a life expectancy of 32.6 years (living to 75.4 years), compared to 40.2 years (living to 83.0 years) in the equivalent age non-diabetic population, corresponding to a mean LLY of 7.6 for the average person with T1DM.
The model showed the ‘average’ person with T2DM (age 65.4 years) has a life expectancy of 18.6 years (living to 84.0 years) compared to the 20.3 years (living to 85.7 years) for the equivalent non-diabetic population, corresponding to LLY of 1.7 years for the average person with T2DM.
Compared with the average LLY for men, the average LLY/person were 21 per cent higher for women with T1DM and 45 per cent higher for women with T2DM.
The authors also add that the NDA reports that 70 per cent of patients with T1DM and 33 per cent of patients with T2DM had a glycated haemoglobin (HbA1c) higher than 58mmol/
mol, so were at higher risk of poor outcomes.
By allocating total LLY to the future life expectancy of both T1DM and T2DM at-risk group, the model shows that each year that a person with either type of diabetes spends with HbA1c >58mmol/mol could shorten their life by 100 days. The authors said:
“Knowledge of this may act as an incentive for clinicians to ensure that all people are on the best therapy to keep their blood sugar in the target range, and for those people to engage more strongly with their therapy and lifestyle recommendations.”
The authors mention some limitations to their study, namely that is used nationallevel mortality data and not GP practicelevel data. Also, it is likely that other factors contribute such as smoking, inactivity, overweight, hypertension and taking of statins.
These will be the subject of a future full analysis with general practice-level data. However, the authors say it is likely that the HbA1c level will remain a strong independent determinant of mortality. ■
Study shows that analysing tears could allow for non-invasive diabetes blood glucose monitoring
A new study presented at this year’s Annual Meeting of EASD shows that analysing the tears of people with diabetes could be a potential method to monitor their blood glucose levels without the need for invasive alternatives that rely on blood testing.
Previous studies of glucose levels in tears have shown that they correspond well to the amount of glucose in the blood. In this research, the Japanese team focused on glycoalbumin (GA) levels, which reflect an average of blood glucose levels over the preceding two weeks; and investigated the
correlation between GA level in tears and those in blood.
A total 100 subjects with diabetes were recruited for the study, and samples of both tears and blood were collected for analysis. Samples from 99 of the 100 participants were successfully measured, and significant correlation was found between GA levels in tears and those in blood. Statistical analysis showed that this correlation was maintained even after adjustment for age, gender, kidney function, and obesity.
Given the strong correlation between GA levels in tears with those in the blood, the team suggest that it has potential to be used as a method of non-invasive diabetes monitoring. They note that since the GA value is expressed as a ratio, the concentration or dilution of tears is not thought to influence the measurement.
The authors said: “In the future, we plan to optimise measurement conditions and develop measurement equipment, and to verify the e ectiveness and usefulness of diabetes monitoring methods.” ■
3 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
Study suggests average person with type 1 diabetes will live almost eight years less and those with type 2 diabetes almost two years less
What have we learned from Covid-19 in persons with type 1 diabetes?
While diabetes is established as a risk factor for severe SARS-CoV2 infection several important specific aspects need to be considered for people with type 1 diabetes. In contrast to older persons with diabetes, children, adolescents and young adults with type 1 diabetes are not at risk for unfavourable outcomes.
However, in a special session on Covid-19 at this year’s Annual Meeting of EASD, Prof Catarina Limbert of the University Centre of Central Lisbon and Hospital Dona Estefania, Lisbon, Portugal, presented new review of viruses that are known to contribute to the new onset of type 1 diabetes and new evidence that SARS-CoV2 infection needs to be added to this list.
“Until now, larger multicentre studies did not find a rise in the number of new cases during the pandemic months compared to the same period in years before,” she explained. “Nevertheless, the Covid-19 crisis has increased the severity at onset of type 1 diabetes with a doubling of people being admitted with diabetic ketoacidosis during the lockdown.”
A population study of 23,804 Covid-19 related deaths in England during 1 March - 1 May 2020 revealed that the odds of dying in hospital with Covid-19 was higher in people with type 1 diabetes (3.5 times) compared to type 2 diabetes (two times). However, the average age at death was 78 years in type 2 diabetes and 72 in type 1 diabetes. It appears, that in type 1 diabetes, only older people aged over 50 years with longer duration of the disease (80 per cent with more than 15 years of disease) and worse glucose control (glycated haemoglobin/ HbA1c >10 per cent) are at higher risk of severe clinical outcomes of Covid-19.
Moreover, according to an early report by the US Centers for Disease Control and Prevention (CDC) from the US with data from 149, 082 Covid-19 cases, only 1.7 per cent were among children <18 years. Diabetes was not among the comorbidities, indicating that with or without diabetes, young people are coping better with Covid-19 infection. Di erences in the anatomy, epidemiology and gene expressions are some of the reasons for the low prevalence of Covid-19 infection in children.
In contrast to challenges related to disease severity and outcomes in type 1 diabetes, the pandemic also o ers opportunities for improving diabetes care management, Prof Limbert said.
“The Covid-19 crisis was the booster shot to put telemedicine into practice. During the lockdown period, healthcare delivery had to adapt and make a sudden transition to remote care. In paediatric diabetes, digital revolution in type 1 diabetes management already started many decades ago with pumps and now extended to integration of sensors, automated insulin delivery or dosage advisors.
“The need to upload the data for a meaningful telemedicine consultation has motivated families to become more involved with digital diabetes data. The use of remote healthcare promoted autonomy of both young people and their parents in interpreting the data and making decisions. Thus, challenges of Covid-19 turned out to be an opportunity for empowerment of people with type1 diabetes.” ■
Study reveals type 2 diabetes remission can restore pancreas size and shape
In 2019, research revealed that achieving remission of type 2 diabetes by intensive weight loss can restore the insulinproducing capacity of the pancreas to levels similar to those in people who have never been diagnosed with the condition. Now, new research presented at the 2020 Annual Meeting of EASD demonstrates for the first time that reversing type 2 diabetes can also restore the pancreas to a normal size and shape.

“Our previous research demonstrated the return to long-term normal glucose control, but some experts continue to claim that this is merely ‘well controlled diabetes’ despite our demonstration of a return to normal insulin production by the pancreas. However, our new findings of
major change in the size and shape of the pancreas are convincing evidence of return to the normal state,” said Prof Roy Taylor from Newcastle University, UK, who led the research.
Previous imaging studies have shown reduced size and abnormal shape of the pancreas in people with type 2 diabetes. But whether these abnormalities resulted from, rather than led to, the disease state was unknown until now.
In the study, 64 participants from the landmark Diabetes Remission Clinical Trial (DiRECT) and 64 age-, sex-, and weight-matched controls without type 2 diabetes were measured over two years for pancreas volume and fat levels, and
irregularity of pancreas borders using a special MRI scan. Beta cell function was also recorded. Responders were classified as achieving a glycated haemoglobin A1c (HbA1c) level of less than 6.5 per cent and fasting blood glucose of less than 7.0mmol/l, o all medications.
At the start of the study, average pancreas volume was 20 per cent smaller (64cm3 vs 80cm3), and pancreas borders more irregular, in people with diabetes compared with controls without diabetes.
After five months of weight loss, pancreas volume was unchanged irrespective of remission (63cm3 to 64cm3 for responders and 59cm3 to 60cm3 in non-responders). However, after two years, the pancreas had
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 4
grown on average by around one-fifth in size (from 63cm3 to 76cm3) in responders compared with around a twelfth (from 59cm3 to 64cm3) in those who did not.
In addition, responders lost a significant amount of fat from their pancreas (1.6 per cent) compared with non-responders (around 0.5 per cent) over the study period, and achieved normal pancreas borders.
Similarly, only responders showed early and
sustained improvement in beta-cell function. After five months of weight loss, the amount of insulin being made by responders increased and was maintained at two years, but there was no change in non-responders.
“Our findings provide proof of the link between the main tissue of the pancreas which makes digestive juices and the much smaller tissue which makes insulin, and open up possibilities of being able to predict future onset of type 2 diabetes by
scanning the pancreas,” said Prof Taylor.
“All our research has been focused on type 2 diabetes which has developed within the last six years. Although some people with much longer duration diabetes can achieve remission, it is clear that the insulin producing cells become less and less able to recover as time passes. We need to understand exactly why this is and find ways to restore function in long duration type 2 diabetes.” ■
Special safety protocol successfully allowed pilots with diabetes to work
A new study presented at this year’s Annual Meeting of EASD shows that the introduction of a new safety protocol has successfully enabled people with insulintreated diabetes to work as commercial pilots, and could potentially allow individuals with the condition to perform other ‘safety-critical’ jobs such as bus drivers or maritime workers.

The study, conducted by Dr Gillian Garden and colleagues at the Department of Metabolism and Ageing, University of Surrey, Guildford, UK, as well as researchers and industry professionals from universities and civil aviation authorities in the UK, Ireland, and Austria, evaluated the performance and safety impact of a new protocol that enabled certificated pilots with insulin-treated diabetes to fly commercial aircraft for the first time.
The risk of hypoglycaemia in people with insulin-treated diabetes has for many years debarred them from working in certain ‘safety-critical’ jobs, including flying commercial airliners.
The UK, together with Ireland and Austria, introduced a ground-breaking safety protocol for certificated pilots with insulintreated diabetes, and now have the largest number of people in the world with the condition working as commercial pilots. Anyone with diabetes is subjected to strict oversight including glucose monitoring
during duty periods, and frequent clinical health reviews.
The team performed an observational study of 49 pilots with insulin-treated diabetes who had been granted medical certification to fly commercial (Class 1 certificate) and non-commercial (Class 2 certificate) aircraft. Clinical details, pre and in-flight (hourly and 30 minutes pre-landing) blood glucose values were compared with the protocolspecified ranges: ‘Green’ (5-15mmol/L), ‘amber’ (low 4-4.9mmol/L, high 15.1-20mmol/L), and ‘red’ (low <4.0mmol/L, high >20.0mmol/L).
In the case of a ‘red’ low reading for example, the pilot is required to immediately hand over duties to the co-pilot or, if flying solo, consider landing as soon as is practical. They must also consume 10-15g of readily absorbed carbohydrate and re-test their blood sugar level after 15 minutes.
Participants in the study had either type 1 (84 per cent) or type 2 (16 per cent) diabetes and had been issued with Class 1 (61 per cent), or Class 2 (39 per cent) medical certificates. Most were male (96 per cent), with a median age of 44 years, a median diabetes duration of 10.9 years, and a median follow-up period of 4.3 years after the receipt of their medical certificate.
Pilots had a mean HbA1c level of 55.0
mmol/mol (7.2 per cent), and a postcertification mean of 55.1 (7.2 per cent). A total of 38,621 blood glucose measurements were taken during 22,078 flying hours, of which 97.69 per cent were within the green range, 1.42 per cent within the low amber range and 0.75 per cent within the high ‘amber range. Only 0.12 per cent of measurements fell within the low ‘red’ range, and just 0.02 per cent were within the high ‘red’ range.
Out of range readings declined from 5.7 per cent in 2013 to 1.2 per cent in 2019, while no episodes of pilot incapacitation occurred and none of the study participants showed a deterioration of their glycaemic control during the 7.5 years of the study.
Use of a ‘tra c light’ system provided a straightforward way of alerting pilots of the need to take preventive action to avoid impairment of performance or decision making that could arise from unduly high or low blood glucose levels.
The authors conclude that the protocol is practical and feasible to implement and has performed well. There were no reports of pilot incapacitation during flights, and no events occurred in which safety was compromised. They point out that this study represents the most extensive data set for people with insulintreated diabetes working in a ‘safetycritical’ occupation. ■
5 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
Insulin pump therapy uptake low in Ireland due to lack of standardised criteria and care
AUTHOR: Priscilla Lynch
New Irish research has identified that insu cient structure of the health service, lack of awareness and individual preferences are among barriers for adults with type 1 diabetes in getting insulin pump therapy. The study, led by researchers from RCSI University of Medicine and Health Sciences, is published in a recent edition of Acta Diabetologica
This research builds on previous studies by RCSI researchers, which showed that people with type 1 diabetes in Ireland use insulin pump therapy at lowered rates compared internationally and there is an unequal availability of insulin pump therapy in Irish adult diabetes clinics.
Even though insulin pump therapy is recommended as a first-choice therapy for pre-schoolers and is beneficial for people with type 1 diabetes of all ages, only 10.5 per cent of people with type 1 diabetes in Ireland are using a pump to administer their insulin. This compares significantly lower to the average uptake in Nordic, central, and western countries, which was 15–20 per cent in 2010.
Regional disparities in insulin pump use in Ireland were found, with uptake as low as 2 per cent among adults in Roscommon compared to 9.6 per cent in Kildare. One-third of Irish adult clinics, usually in rural areas, do not o er any type of insulin pump therapy support, and less than half provide training to commence the therapy.
To identify the reasons for why uptake is lower in Ireland, the researchers conducted 21 interviews and four focus groups among people with type 1 diabetes, healthcare professionals and other key stakeholders.
The same topics were discussed in all groups and aligned with four main themes: awareness, structure, capacity and impact of an individual (a person with diabetes or healthcare
professional). The main finding was that if the structure of the health service is insu cient, the quality of care is not standardised and capacity is poor, the uptake of insulin pump therapy is more reliant on the individual’s interest, leadership skills, willingness and motivation.
“These factors may make the regional di erences in accessing diabetes related technology and the quality of care more evident,” said Dr Katarzyna Gajewska, the study’s lead author and HRB PhD scholar in population health and health service research
NUI GALWAY SEEK PARTICIPANTS FOR DIABETES PREVENTION STUDY
Researchers from the School of Psychology in NUI Galway are inviting people to share their views on diabetes, diet, physical activity, and a programme that uses a smartphone app and live health coaching to help people improve their health. The PRE-T2D (Prevention of Type 2 Diabetes) study is a 15-minute online survey that is open to all people aged over 18 living in Ireland and ndings will be critical to the development of an online diabetes prevention programme to be delivered in Ireland.
Luke Van Rhoon, PhD Candidate in Health Psychology, Health Behaviour Change Research Group, NUI Galway, said: “We aim not only to prevent diabetes, but help people to better manage their diet, exercise, and daily stress in the long run. This is particularly important as we currently face many new physical and psychological challenges due to the emergence of Covid-19.
“Technology is becoming increasingly vital in the self-management of our health and how we communicate with healthcare professionals, friends, and family. Although
at RCSI. “The results of this study may inform healthcare professionals and policy makers regarding gaps in the delivery of diabetes care. Solutions are needed to reduce the disparities in health service provision in the countries where reimbursement of diabetes technology is o ered. Such steps may include the development of national guidelines, models of care, and structured approaches to provide equal access to insulin pump therapy across the country.”
Study link: https://link.springer.com/article/10.1007 per cent2Fs00592-020-01595-5
online diabetes prevention programmes have been successfully implemented in other countries, it is important to create a unique programme that suits the needs of the Irish population.”
This study is funded by the Irish Research Council and is supervised by Prof Molly Byrne and Dr Jenny McSharry, Directors of the Health Behaviour Change Research Group at NUI Galway. Prof Byrne said online programmes can overcome some of the challenges a ecting face-to-face programmes, “and we now know from the research that digital health interventions can be e ective in increasing physical activity, changing diets and promoting weight loss. Our research, which is being conducted in collaboration with the National Programme for Diabetes, will provide really important ndings to ensure that online diabetes prevention programmes which are developed in Ireland are usable by the people who will bene t most from them.”
For more information about the PRE-T2D study visit, www.pret2d.com/survey or contact Luke Van Rhoon at l.vanrhoon1@ nuigalway.ie
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 6
The 1st polypill licensed for secondary prevention of cardiovascular events in Ireland













Thanks to you...














Aspirin Atorvastatin Ramipril







6 Available Formulations
























Reduce pill burden by 730 pills per year*
Capsules shown are not actual size. Capsules are Size 0. Please refer to SmPC before prescribing.




*The calculation of a reduction of 730 pills per year is based on a patient taking the three individual components of Trinomia (aspirin, atorvastatin, ramipril) on a daily basis for 365 days compared to a patient taking a Trinomia capsule once daily for 365 days.



Trinomia 100 mg/20 mg/10 mg, 100 mg/20 mg/5 mg, 100 mg/20 mg/2.5 mg hard capsules (acetylsalicylic acid, atorvastatin (as atorvastatin calcium trihydrate) and ramipril) and Trinomia 100 mg/40 mg/10 mg, 100 mg/40 mg/5 mg, 100 mg/40 mg/2.5 mg hard capsules (acetylsalicylic acid, atorvastatin (as atorvastatin calcium trihydrate) and ramipril) Abbreviated Prescribing Information Please consult the Summary of Product Characteristics (SmPC) for full prescribing information. Presentation: Hard capsules containing: two 50 mg acetylsalicylic film-coated tablets, two 10 mg atorvastatin film-coated tablets and one 10 mg ramipril film-coated tablet; or two 50 mg acetylsalicylic filmcoated tablet, two 10 mg atorvastatin film-coated tablets and one 5 mg ramipril film-coated tablet; or two 50 mg acetylsalicylic film-coated tablet, two 10 mg atorvastatin film-coated tablets and one 2.5 mg ramipril film-coated tablet; or two 50 mg acetylsalicylic film-coated tablets, two 20 mg atorvastatin film-coated tablets and one 10 mg ramipril film-coated tablet; or two 50 mg acetylsalicylic film-coated tablet, two 20 mg atorvastatin film-coated tablets and one 5 mg ramipril film-coated tablet; or two 50 mg acetylsalicylic film-coated tablet, two 20 mg atorvastatin film-coated tablets and one 2.5 mg ramipril film-coated tablet. Uses: Secondary prevention of cardiovascular accidents as substitution therapy in adult patients adequately controlled with the monocomponents given concomitantly at equivalent therapeutic doses Dosage: Oral administration. 1 capsule per day, preferably after a meal. Swallow with liquid. Do not chew or crush. Avoid grapefruit juice. Patients currently controlled with equivalent therapeutic doses of acetylsalicylic acid, atorvastatin and ramipril can be directly switched. Treatment initiation should take place under medical supervision. Cardiovascular prevention, target maintenance dose of Ramipril is 10 mg once daily. Daily dose in renal impairment based on creatinine clearance - ≥ 60 ml/min, maximum daily dose is 10 mg ramipril; 30-60 ml/min, maximum daily dose is 5 mg ramipril. Contraindicated in hemodialysis and/or with severe renal impairment (creatinine clearance <30 ml/min). Administer with caution with hepatic impairment. Perform liver function tests before initiation of treatment and periodically thereafter. Maximum daily dose is 2.5 mg ramipril and initiate treatment under close medical supervision. Contraindicated in severe or active hepatic impairment. Start treatment in very old and frail patients with caution. In patients taking elbasvir/grazoprevir concomitantly with atorvastatin, the dose of atorvastatin should not exceed 20 mg/day. Contraindications: Hypersensitivity to any component, to other salicylates, to NSAIDs, to any other ACE inhibitors, tartrazine, soya or peanut. History of previous asthma attacks or other allergic reactions to salicylic acid or other NSAIDs. Active, or history of recurrent peptic ulcer and/or gastric/intestinal haemorrhage, other kinds of bleeding. Haemophilia and other bleeding disorders. Severe kidney and liver impairment. Hemodialysis. Severe heart failure. Concomitant treatment with methotrexate at a dosage of 15 mg or more per week. Concomitant use with aliskiren-containing products with diabetes mellitus or renal impairment. Nasal polyps associated with ashma induced or exacerbated by acetylsalicylic acid. Active liver disease or unexplained persistent elevations of serum transaminases. Pregnancy, lactation and in women of child-bearing potential not using appropriate contraceptive measures. Concomitant treatment with tipranavir, ritonavir, ciclosporin, glecaprevir/pibrentasvir,sacubitril/valsartan therapy. Trinomia must not be initiated earlier than 36 hours after the last dose of sacubitril/valsartan. History of angioedema. Extracorporeal treatments leading to contact of blood with negatively charged surfaces. Significant bilateral renal artery stenosis or renal artery stenosis in a single functioning kidney. Hypotensive or haemodynamically unstable states. Children and adolescents below 18 years of age. Warnings and Precautions: Only for use as a substitution therapy in patientsadequately controlled with the monocomponents given concomitantly at equivalent therapeutic doses. Special populations requiring particularly careful medical supervision: Hypersensitivity to other analgesics/antiinflammatory/antipyretic/antirheumatics or other allergens. Other known allergies, bronchial asthma, hay fever, swollen nasal mucous membranes and other chronic respiratory diseases. History of gastric or enteric ulcers, or of gastrointestinal bleeding. Reduced liver and/or renal function. Particular risk of hypotension: strongly activated renin-angiotensin-aldosterone system, transient or persistent heart failure post MI, risk of cardiac or cerebral ischemia, in case of acute hypotension medical supervision including blood pressure monitoring is necessary. Deterioration of cardiovascular circulation. Glucose 6 phosphate dehydrogenase deficiency. Risk of elevated levels of uric acid. Consumption of substantial quantities of alcohol and/or have a history of liver disease. Diagnosed pregnancy, stop treatment immediately, and, if appropriate, start alternative therapy. ACE inhibitors cause higher rate of angioedema in black patients than in non-black patients. The blood pressure lowering effect of ACE inhibitors is somewhat less in black patients than non-black patients. Monitoring during treatment is required for: Concomitant treatment with NSAIDs, corticosteroids, SSRIs, antiplatelet drugs, anticoagulants, ibuprofen. Signs or symptoms suggestive of liver injury. Stop treatment temporarily prior to elective major surgery and when any major medical or surgical condition occurs. Particularly careful monitoring is required in patients with renal impairment, risk of impairment of renal function, particularly with congestive heart failure or after a renal transplant. Serum potassium: ACE inhibitors can cause hyperkalemia in patients with impaired renal function and/or in patients taking potassium supplements (including salt substitutes), potassium-sparing diuretics, trimethoprim or co-trimoxazole and especially aldosterone antagonists or angiotensin-receptor blockers, hyperkalemia can occur. Potassium-sparing diuretics and angiotensin-receptor blockers should be used with caution in patients receiving ACE inhibitors, and serum potassium and renal function should be monitored. Other situations that may increase the risk of hyperkalaemia are: age >70 years, uncontrolled diabetes mellitus, dehydration, acute cardiac decompensation or metabolic acidosis.Specific side-effects: Perform liver function tests before use and monitor periodically and with liver injury or increased transaminase levels. Use with caution with substantial alcohol use or history of liver disease. Potential risk of hemorrhagic stroke. May affect the skeletal muscle and cause myalgia, myositis, and myopathy that may progress to rhabdomyolysis, ask patients to promptly report skeletal muscle effects (muscle pains, cramps or weakness) especially if accompanied by malasie or fever and measure CK levels, stop treatment if significantly elevated or if severe muscular symptoms occur. Prescribe with caution in patients with pre-disposing factors for rhabdomyolysis. Benefit/risk of treatment should be considered and clinical monitoring recommended. Do not measure CK following strenuous exercise or in presence of plausible alternative cause of CK increase. If CK levels significantly elevated at baseline, re-measure levels 5 to 7 days later to confirm the results. Risk of rhabdomyolsis with use of potent CYP3A4 inhibitors, transport proteins or HIV protease inhibitors. Consider alternative treatments if risk of myopathy. Consider lower starting or maximum dose and appropriate clinical monitoring with potent CYP3A4 inhibitors and medicinal products that increase the plasma concentration of atorvastatin respectively. The risk of myopathy may also be increased with the concomitant use of gemfibrozil and other fibric acid derivates, antivirals for the treatment of hepatitis C (HCV) (boceprevir, telaprevir, elvasvir/grazoprevir), erythromycin, niacin or ezetimibe. Do not co-administer with systemic fusidic acid or within 7 days of stopping fusidic acid. Where use of systemic fusidic acid considered essential, discontinue statin treatment during fusidic acid treatment. Reports of rhabdomyolysis in patients receiving fusidic acid and statins in combination. Where prolonged systemic fusidic acid needed, consider need for co-administration of Trinomia and fusidic acid on case by case basis with close medical supervision. Discontinue statin treatment if interstitial lung disease occurs. Monitor patients at risk of diabetes mellitus. Discontinue treatment if angioedema occurs and initiate emergency treatment promptly. Concomitant use of ACE inhibitors with sacubitril/valsartan is contraindicated. due to the increased risk of angioedema. Treatment with sacubitril/valsartan must not be initiated earlier than 36 hours after the last dose of Trimomia. Caution should be used when starting racecadotril, mTOR inhibitors and vildagliptin in a patient already taking an ACE inhibitor as there is an increased risk of angioedema. Concomitant use of ACE-inhibitors and angiotensin II receptor blockers or aliskiren is not recommended and should not be used in patients with diabetic nephropathy. Anaphylactic reactions during desensitization, consider temporary discontinuation of Trinomia during desensitization. Monitor white blood cells for neutropenia/agranulocytosis and more regularly in the initial phase of treatment, impaired renal function, concomitant collagen disease and other medicines that can change the blood picture. Cough. Contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine. Interactions: Acetylsalicylic acid: other platelet aggregation inhibitors, other NSAIDs, and antirheumatics, systemic glucocorticoids, diuretics, alcohol, SSRIs, uricosuric agents, metamizole, anticoagulant and thrombolytic therapy, digoxin, antidiabetic agents including insulin, methotrexate, valproic acid, antacids, ACE inhibitors, ciclosporin, vancomycin, interferon , lithium, barbiturates, zidovudine, phenytoin, laboratory tests. Atorvastatin: CYP3A4 inhibitors, CYP3A4 inducers, transport protein inhibitors, gemfibrozil/fibric acid derivatives, ezetimibe, colestipol, fusidic acid, colchicine, digoxin, oral contraceptives, warfarin. Ramipril: potassium salts, heparin, potassium-retaining diuretics and other plasma potassium increasing active substances, antihypertensive agents and other substances that may decrease blood pressure, vasopressor sympathomimetics and other substances, allopurinol, immunosuppressants, corticosteroids, procainamide, cytostatics and other substances that may change the blood cell count, lithium salts, antidiabetic agents including insulin. Monitor as appropriate. Consider lower maximum dose of atorvastatin with potent CYP3A4 inhibitors. Pregnancy and Lactation: Contraindicated in pregnancy and breast-feeding. Women of child-bearing potential should use effective contraception during treatment. Side Effects: Ramipril: Common (≥1/100, <1/10): dyspepsia, nausea, diarrhoea, vomiting, digestive disturbances, abdominal discomfort, gastrointestinal inflammation, non-productive tickling cough, bronchitis, sinusitis, dyspnoea, headache, dizziness, rash in particular maculo-papular, blood potassium increased, myalgia, muscle spasms, chest pain, fatigue, hypotension, orthostatic blood pressure decreased, syncope. Atorvastatin: Common: dyspepsia, nausea, diarrhoea, constipation, flatulence, pharyngolaryngeal pain, epistaxis, nasopharyngitis, headache, allergic reactions, hyperglycaemia, myalgia, muscle spasms, pain in extremity, joint swelling, back pain, arthralgia, liver function test abnormal, blood creatine kinase increased. ASA: Very Common (≥ 1/10): Gastrointestinal complaints such as heartburn, nausea, vomiting, stomach ache and diarrhea, minor blood loss from the gastrointestinal tract (micro-bleeding). Common: Paroxysmal bronchospasm, serious dyspnoea, rhinitis, nasal congestion. For less frequent side effects see SmPC. Pack Sizes: Blister containing 28 hard capsules. Legal Category: POM. Product Authorisation Numbers: PA 1744/002/001-006 Product Authorisation

aspirin • atorvastatin
AspirinAtorvastatinRamipril 100mg 20mg 2.5mg 100mg 20mg 5mg 100mg 20mg 10mg 100mg 40mg 2.5mg 100mg 40mg 5mg 100mg 40mg 10mg
Holder: Ferrer Internacional, S.A., Gran Vía Carlos III, 94, 08028 Barcelona, Spain. Marketed by: A. Menarini Pharmaceuticals Ireland Ltd. Further information is available on request from A. Menarini Pharmaceuticals Ireland Ltd, 2nd Floor, Castlecourt, Monkstown Farm, Monkstown, Glenageary, Co. Dublin A96 T924 or may be found in the SmPC. Date of Preparation: March 2020 Date of item: July 2020. IR-TRI-05-2020 References: 1. Trinomia 100mg / 40mg / 10mg, 100mg / 40mg / 5mg, 100mg / 40mg / 2.5mg SmPC March 2020 2. Trinomia 100mg / 20mg / 10mg, 100mg / 20mg / 5mg, 100mg / 20mg / 2.5mg SmPC March 2020
Getting diabetes services back up and running during Covid-19
AUTHOR: Paul Mulholland
The Covid-19 pandemic has had significant ramifications for patients with chronic conditions, such as diabetes. Like other parts of the health services, traditional diabetes clinics were paused in the early months of the crisis. During this time, consultations began taking place virtually, with healthcare practitioners providing advice around self-management of the condition.
However, as normal services resumed, some patients with diabetes were still reluctant to attend their hospital appointments as they are in an ‘at-risk’ group in terms of Covid-19 infection. This led the HSE and Diabetes Ireland to issue a call, urging people with concerns about their diabetes to seek medical advice from their pharmacist, GP, or hospital diabetes team.
Speaking to Update, Prof Sean Dinneen, Consultant Endocrinologist at Galway University Hospital (GUH) and Clinical Lead of the HSE National Clinical Programme for Diabetes, said it was important that patients with diabetes were aware of the availability of support and care, despite the pandemic.

“We’ve noticed that patients are not always keen to come to hospital,” stated Prof Dinneen.
“There is a fear of contracting the virus, perhaps reduced in recent weeks, but I suspect starting to ramp up again now. And it is trying, I suppose, to walk that tightrope between providing care and getting services back up and running, and at the same time trying to get people to focus on health management of their diabetes.”
One positive factor of the early lockdown, Prof Dinneen said, is that many people have become more proactive in terms of managing their condition.
However, he added that his “strong impression” from talking to colleagues around the country is that patients are presenting at a later stage with complications arising from their diabetes.
“Patients are not coming at the appropriate time,” he told Update.
“And what we are seeing is later presentations of problems that could have been more easily dealt with, if they
presented earlier. Diabetic foot is a good example. If you don’t come early, the problem can get worse and be more di cult to manage. I think that ophthalmologists are also concerned about patients with visual symptoms presenting later.”
National registry
In the absence of a national register for diabetes, Prof Dinneen said it was di cult to specifically say what impact the pandemic has had on people with the condition.
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 8
There is a fear of contracting the virus, perhaps reduced in recent weeks, but I suspect starting to ramp up again now. And it is trying, I suppose, to walk that tightrope between providing care and getting services back up and running...
Prof Sean Dinneen
“One of my bugbears is a lot of our colleagues across Europe have a better handle of their diabetes population than us,” he commented.
“Without a national diabetes registry, we are really missing out on the opportunity to track how people are doing. If you said to me, what impact did Covid-19 have on people with diabetes in Ireland, the honest answer is I don’t know.”
A Sláintecare-funded project to establish such a registry has been paused as the team behind its development, which mainly consisted of public health doctors and specialists, were redeployed as a result of the pandemic.
“We need to know who has it and what sort of burden of complications and what sort of burden of illness are we dealing with. And we have the wherewithal now, because we have Diabetic RetinaScreen up and running. RetinaScreen is aware of somewhere in the region of 180,000 people with diabetes in Ireland. So we can build on that. And there is also the new chronic disease management GP contract. The plan we have for the Sláintecare project is to merge those two sources of data, and try to establish a proper national diabetes registry over the next few years. I think Covid has highlighted that need even more.”
The redeployment of healthcare sta from various parts of the health service to assist in the fight against the pandemic has impacted on diabetes teams, according to Prof Dinneen. He said HSE management has been informed about the need for sta to return to diabetes services.
“And I think that message has gotten across,” he said.
“However, we still don’t have a full complement of our community podiatrists here in CHO 2” [Community Healthcare Organisation, Area 2, which covers Galway, Roscommon, and Mayo].
Prof Dinneen said that his department in GUH reached out to all patients who were booked to have a face-to-face appointment.
“Not every department around the country was able to do that, but we did, mainly by phone,” he said.
“We are only beginning to embrace the world of video conferencing with our patients. I know other departments have already got up and running with that function. We have used it mainly for our own internal meetings. And teaching and things like that are resuming now, with Zoom and Microsoft Teams. But in our own case here in Galway it is mainly phone contact with patients that we’ve used. Attend Anywhere and other HSE-supported software are coming into the frame now and people are beginning to use that telemedicine or video conferencing with patients.”
Telemedicine consultations are more suitable for patients who have previously attended the clinic, according to Prof Dinneen. He added that younger patients,
in particular, value this type of consultation.
“We would have a very high non-attendance rate in our young adult clinics in general. For whatever reasons they don’t turn up,” he said.
“We had almost zero non-attendance because we could connect in with them remotely. And they love it.”
However, he said for patients attending for the first time, or for complicated cases, face-to-face interactions were vital, even if telemedicine had the potential to “change practice”.
“If it is a straightforward visit, we should and we will in the future, do it remotely,” he said.
“The challenge is identifying the straightforward visit. Because sometimes a visit you think will be straightforward turns out to be anything but.” ■
HSE LAUNCHES LIVING WELL FREE ONLINE PROGRAMME SUPPORTING PEOPLE WITH LONG-TERM HEALTH CONDITIONS DURING COVID-19
The HSE National Self-Management Support team recently o cially launched the Living Well Programme, a series of online workshops designed to o er support to people living with long-term health conditions, including diabetes.
Living Well is a free group programme which runs online for six weeks. There is one workshop a week, which lasts 2.5 hours. Workshops are delivered in a relaxed and friendly way so that all participants can learn from each other. There are a maximum of 12 people in a programme. Two trained facilitators run the workshops each week. At least one of the facilitators lives with a long-term health condition.
The Living Well Programme has received Sláintecare integrated funding to enable delivery during 2020/2021. The programme was previously delivered in a face-to-face community setting, but it has been made available online during the
Covid-19 pandemic.
Dr Derval Howley, Chair of Interim HSE National Advisory Group for Self Management Support, said: “People taking part in the workshops may have di erent health conditions, however, they all face similar challenges - for example managing medications, attending healthcare appointments, communicating with healthcare professionals, coping with pain, fatigue, and di cult emotions. Living Well is a fantastic free programme which builds con dence and helps participants develop the skills required to better manage their conditions in a supportive and safe way.”
Anyone with a long-term health condition interested in taking part in this programme can register by contacting their local Living Well Team, contact details of which are available at www.hse.ie/livingwell, or contact HSELive on 1850 24 1850 / 01 240 8720 or email hselive@hse.ie.
9 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
Is obesity really a disease?
AUTHORS: Dr Finian Fallon and Prof Carel le Roux, Obesity Complications Clinic, St Vincent’s Healthcare Group, Dublin
Since the World Health Organisation (WHO) recognised obesity as a chronic disease in 2000, there has been a slowly building consensus in healthcare that obesity can or should be treatable within the medical paradigm. Despite growing recognition that obesity is a complex phenomenon, the idea that obesity is a kind of moral choice remains somewhat ingrained in our culture, including among many of those who are living with obesity and among many healthcare professionals who care for patients with obesity.
While changes around food intake and lifestyle choices related to obesity remain relevant interventions, from a scientific perspective increasingly the evidence is demonstrating that obesity is a biologically embedded process. As such, we are seeing that the solution for the epidemic of obesity, if there is one, is increasingly outside the realm of willpower and moral fibre. Instead, research is proving that appropriate treatment trajectories reside in our medically grounded understanding that, similar to cancer not being one uniform disease, obesity appears to have many subsets of disease all contributing to excess adipose tissue. While treatment vectors may include food and lifestyle choices, these elements of our interventions are not e ective for all forms of obesity. This is often contrary to the lay public, and often health professionals’ beliefs. The ‘eat less, move more’ message has sustained a huge and growing worldwide diet industry for many decades and is an implicit position in many encounters between patients and medical interventionists. However, the science has shown that for most forms of obesity this message simply is not enough.
Blame culture
In a recent study carried out by Grannell et al (2020) we see how the blame culture that surrounds obesity can lead to
stigma and a reduction in health-seeking behaviours among those with obesity. In this study of 52 patients with obesity, contradictory perspectives emerge about patient beliefs and perceptions around obesity as a disease, its treatment and its causes. Patients in the study tended to agree with an apparently contradictory belief: while agreeing that obesity is a disease, they tend to agree that its solution is related to willpower. In essence, as the study finds, “there is a discrepancy between recognition that obesity is a chronic disease and treating obesity as a chronic disease.” This “misalignment” may perpetuate stigma, and impacts healthseeking behaviours and engagement with services when help is sought. Patients also had conflicting views on the relevance and importance of exercise to weight loss. Again, the prevailing view that exercise can have a significant impact on weight loss may be true for a few forms of obesity but increasingly its benefit is seen in the aftermath of weight loss during the weight loss maintenance period.
The message that Grannell et al are o ering in this research is that there is a pressing need to move from a focus on weight loss alone, to one which is primarily concerned with health gain. They argue that this message is part of a necessary reframing of obesity requiring treatment like any other chronic disease. This would still include exercise and food intake as an element, but does not have as its main focus the stigmatising and generally ine ective ‘eat less, move more’ refrain that echoes around the world of weight loss. This health gain focus is essentially, they argue, one which is grounded in an understanding of obesity as a disease which recognises the biological determinants of obesity, albeit embedded in a complex array of forces which are internal and external to the individual.
Covid-19
Covid-19 has added another layer of complexity to our response and understanding of the disease of obesity. Grannell et al (2020) also carried out a qualitative study into the fears of those with obesity during the pandemic. Their findings indicate that those with obesity are at risk of additional weight gain and increased existential anxiety in the context of under-resourced obesity services. Patients are reporting challenges in accessing services, maintaining routine and exercising. These are challenges that speak to the need for careful consideration of the mental health complications associated with obesity and the experience of stigma. There is a risk of a two-tiered society developing, between the haves and have nots of those living with obesity and those with less visible conditions, with one group facilitated to return to normal living in spite of the risks of developing Covid-19, while another group of those with obesity are at risk of experiencing the fear and stigma that confronts those with this often visually apparent disease. Professionals are under an onus to ensure that public debate is sensitive to the risk of ‘othering’ those with obesity, in addition to the need for increasing awareness of the benefits of additional resourcing for obesity services. This is not to privilege obesity, but seeks to recognise the public health and societal benefits of appropriate and thorough recognition of obesity as a disease that can be treated.
Future advances
The science of endocrinology and obesity has progressed rapidly in recent years. While di erent fields have made great claims in relation to the possibilities of scientific knowledge, which ultimately prove overly confident, the next 10 years in the field of medicine may well o er a novel gateway into the world of personalised treatments for obesity. It may be that the utilisation of artificial intelligence to help
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 10
manage the complex balancing of hormonal and other biological and neurological processes will lead to more widely successful, truly individualised obesity treatments. These interventions may well incorporate more focused surgical procedures or medical devices with enhanced medications.
Conclusion
In conclusion, the science which focuses
on obesity as a disease and one which is focused on health gain is more e ective than the one which dominates the prevailing discourse around obesity. The prevailing discourse is one which perpetuates unconscious biases, often contained in well-intentioned messages, among both patients and health professionals. By declaring obesity a disease, we are giving agency to patients, but also to
healthcare professionals. Our healthcare systems are getting better all the time in managing chronic diseases. Our task is to ensure that obesity is accepted and treated as one of those diseases and apply the same processes and level of acceptance that we would for any other complex and chronic disease. ■
References on request
Maintaining obesity services amid Covid-19
AUTHOR: Priscilla Lynch
The Irish Society for Clinical Nutrition and Metabolism (IrSPEN) has cautioned that patients with obesity are at higher risk of complications from Covid-19 – and need equal access to treatments as the health system begins to address backlogs and schedule new appointments.
To help manage this challenge, obesity consultants from around the world have together published guidance on prioritising access to surgery and treatments for those with obesity and associated diabetes.
Tallaght University Hospital Consultant Endocrinologist, Dr Conor Woods stated: “Similar to most elective surgery, metabolic procedures have been postponed during the pandemic. However, due to the progressive nature of diabetes, delaying surgery can increase future health complications and even earlier death.
“The traditional ‘weight-centric’ criteria for patient prioritisation needs to change. For the period ahead, a new triaging approach for obesity and diabetes surgeries and treatments has been agreed internationally.”
Guidelines
If patients are well enough to be safe surgical candidates, preference should be a orded to those with the greatest risk of morbidity and mortality from their disease, if it is probable that this risk can be reduced by surgery, the new guidelines note. This logic would apply, for instance, to many surgical candidates
with poorly controlled type 2 diabetes or substantial metabolic, respiratory, or cardiovascular disease.
Traditional BMI-centric criteria for patient selection, however, tend to skew access to bariatric and metabolic surgery in the opposite direction. Despite strong evidence that surgery achieves its greatest health benefits among patients with type 2 diabetes, a minority of those who have such operations have preoperative type 2 diabetes or cardiometabolic disease, the guidelines state.
Furthermore, the document notes that in many publicly funded healthcare systems, candidates for bariatric and metabolic surgery are currently placed on a single elective surgery waiting list, regardless of their indication. Priority is established largely on a first-come first-served basis, rather than on clinical need. “This approach is comparable to putting all colorectal surgery candidates on the same waiting list with similar priority, regardless of whether their diagnosis is cancer or benign neoplasia. A strong need therefore exists for clinically sound criteria to help prioritise access to surgery in times of pandemics with limited resources. These criteria can also inform future waiting list management and decision making about the structure of surgical services,” the guidelines say.
The guidelines recommend that patients be prioritised into three categories:
▸ Surgery within 30 days for those who have complications of previous metabolic surgery.
▸ Surgery within 90 days for patients with substantial risk of complications of diabetes or who have poor control of their diabetes, despite complex medical regimens or using insulin.
▸ Standard access to surgery for patients who are unlikely to deteriorate within six months, but these patients need to be optimised using intensive medical treatment.
Given the risks of severe complications from Covid-19 in patients with diabetes and obesity, the recommendations include that Covid-19 screening be mandatory prior to any obesity treatment, that keyhole surgery remains the best approach and that personal protective equipment (PPE) should be used.
IrSPEN member and Metabolic Surgeon Prof Helen Heneghan added: “Although we will be particularly focussed on how we should restart activity in the immediate post-Covid-19 period, the new guidelines also provide a framework for clinical prioritisation long into the future.”
James Cushnan, a patient from Letterkenny with diabetes who had his metabolic surgery postponed due to Covid-19, added: “Doctors, policymakers, and hospital managers must recognise the seriousness of diseases that require metabolic surgery and ensure these operations are not further delayed due to the widespread misconception that obesity and diabetes are lifestyle conditions of laziness and that surgery is a ‘last resort’.” ■
11 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
Advances in diabetes technology
AUTHOR: Dr Anna Clarke, Advocacy and Research Manager, Diabetes Ireland
Diabetes is a serious health issue with approximately 225,000 people diagnosed in Ireland, which poses a challenging problem for acute and community services. Acute services are required to provide care for all people with type 1 diabetes and all complicated type 2 cases, leaving the remaining 100,000 people to get routine care at community level. Diabetes care has advanced with more options for medical management, increased emphasis on self-management, improved technology solutions for glucose monitoring and delivery of insulin, but the focus of care remains on optimisation of glucose, cholesterol and blood pressure to reduce diabetes complications. For most people this requires daily decision making regarding food intake, medication and activity balance and for many a continuous struggle between hyperglycaemia and hypoglycaemia.
Diabetes technologies have improved health outcomes; eg, pump technology compared to multiple daily injections improves glycaemic control and gives greater flexibility in daily life.
Technology advances o er motivated individuals more precise dosage regimes and glucose fluctuation information thereby giving enhanced safety and discretion. However, technology may increase diabetes burden with di erent age groups perceiving di erent advantages and disadvantages to its usage.
This article focuses on devices used for insulin administration, glucose measurement and assisted insulin adjustment currently available in Ireland, acknowledging that access to newer technologies is limited by cost and personal factors. This article is relevant to 2020 only as technology advances happen rapidly with new iterations happening annually.
Insulin administration
Traditionally, syringes/vials were used to administer insulin but now insulin pens are the most widely used devices for delivering insulin. Continuous insulin administration, ie, insulin pumps are computer assisted methods of insulin administration introduced in the 1970s. Gajewska et al (2020) reported that 2,111 people in Ireland are using insulin pumps with a five-fold increased prevalence among children and adolescents. Insulin pumps are similar in size to a mobile phone and programmed to release doses of insulin continuously (basal), or as a surge (bolus) to control blood glucose levels post carbohydrate consumption. The basal rates are pre-programmed or automatically calculated by the pump. Bolus doses are calculated by the individual (technology can assist) in response to carbohydrate consumed or to correct a high glucose reading. As a general rule, approximately 50 per cent of the total daily insulin dose is used as basal insulin and 50 per cent as bolus insulin. A reservoir is filled with rapid-acting insulin and connected to an infusion set inserted into the subcutaneous tissue and renewed every two-to-three days. Hence, these devices are attached to the person continuously.
Sensor augmented pumps such as the Medtronic Veo, used with appropriate glucose sensor technology, allow the setting of alarms at low glucose levels to trigger insulin cut-o . Newer pumps allow for predictive low glucose suspend, ie, Medtronic 640G uses an algorithm to predict that a low glucose level will occur within 30 minutes and suspends insulin delivery. The Medtronic 670G uses hybrid closed-loop technology, which involves complex predictive algorithms to automatically increase, decrease or suspend insulin delivery to keep glucose levels at a specific target glucose of 6.7mmol/L,
e ectively minimising hyperglycaemia and hypoglycaemia. However, unless in complete automatic mode, carb counting and appropriate bolus insulin are required. It is expected that the Medtronic 780G will be available soon in Ireland.
PEOPLE USING PUMPS EMPLOY TERMINOLOGY
SUCH AS:
▸ Insulin to carbohydrate ratio (ICR): the amount of carbohydrate consumed that requires one unit of insulin to maintain glucose levels.
▸ Insulin sensitivity factor (ISF): the e ect one unit of insulin has in lowering glucose levels.
▸ Insulin on board/active insulin: the amount of insulin still active in the body from a previous bolus. It is essential to know this when calculating the next bolus to reduce the risk of insulin stacking and hypoglycaemia.
There are risks associated with pumps including pump failure, line occlusions, infusion site reactions/infection or fat accumulation, ie, lipohypertrophy. These episodes are not infrequent and education regarding troubleshooting and vigilance is required to avoid acute hyperglycaemia and diabetic ketoacidosis.
Glucose monitoring
The most common method of glucose monitoring for people with diabetes requiring home glucose checks remains the home blood glucose meter (HBGM) or spot glucose testing. A finger prick is undertaken and blood applied to a test strip inserted into a meter, which gives a visual or audible reading in five-to-15 seconds. Measurement of glucose levels allows the person to recognise out-ofrange results and take action, such as selecting an appropriate dose of insulin
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 12
or carbohydrate intake or implement dietary or other lifestyle changes. Each person in conjunction with their diabetes team devises a frequency of testing and targets to be achieved with a detailed plan of action for out-of-range levels. Many types of portable blood glucose meters are available, from basic models to moreadvanced meters with multiple features and options. More recent developments are the advent of the finger prick being replaced by sensors.
Continuous glucose monitoring (CGM) requires a small electrode (sensor) to be inserted under the skin which measures interstitial fluid glucose levels every five minutes and displayed on a device/reader or a smartphone/watch. An alarm can be set to alert of glucose levels that are too low or too high or the data can be linked to an insulin pump.
Flash glucose monitors (FGM) requires a sensor applied on the skin, which measures interstitial glucose levels every five minutes and is stored in the sensor to be read by a reader or smartphone app. The display shows a trend arrow indicating if glucose levels are rising or falling but does not allow for alarm settings or linking to pump therapy yet. Trends highlight the direction that glucose readings are moving and the speed at which they are changing. Trends alert the individual if glucose levels have been rising, falling, or appear to have been stable over several minutes/ hours as opposed to finger stick readings or
individual sensor readings which are only snapshots of glucose levels at that moment. The data available through CGM and FGM can permit significantly more fine-tuned adjustments in insulin dosing and other therapies than HBGM can provide. With nearly 300 measurements a day, CGM/FGM systems o er a new way to evaluate diabetes management by exploring the percentage of the time the person’s glucose readings are within target range: Time in range. For most people, their aim should be to have glucose levels within target range for >70 per cent of the time with less stringent targets for older or high-risk individuals and for those under 25 years of age.
It is important to remember that CGM and FGM measure interstitial glucose levels and that there is a five-to-10 minute delay in interstitial glucose response to changes in blood glucose. Thus, data from a glucose meter is not interchangeable with CGM/ FGM data. Similarly, the trend arrows in CGM/FGM are not interchangeable; refer to individual device manual for interpretation.
Assisted insulin adjustment
The majority of people using insulin to manage their diabetes (almost all people with type 1 diabetes) will calculate their insulin requirement based on their current glucose level and how much carbohydrate they are having in a meal. This is a complex calculation requiring:
▸ knowledge of the carbohydrate content of food to be consumed;
▸ calculate insulin requirement according to individual ICR (may di er based on time of day or month for females);
▸ include extra insulin according to ISF if glucose level above target;
▸ adjust for any active insulin in body;
▸ adjust for planned exercise.
When calculated this is the required insulin dosage. Newer smart meters will allow input of the required data (ICR and ISF can be pre-programmed) and will calculate and record how much insulin is recommended thus minimising mathematical errors.
Conclusion
Advances in technology are changing everyone’s life and while technology can admittedly be helpful it may also burden us with increased engagement; additional information and a constant strive to do better. For people with diabetes the same applies: Advances have reduced the burden of diabetes physical management but at a cost to emotional burden and daily quality-of-life. Technology works for some people but uptake of these systems has been limited by Government policy, financial cost, and individual factors both of the patient and the professional. While there is great interest in new technology, it is important to continue to make best use of available resources to improve outcomes for people with diabetes which currently remain suboptimal mainly due to inadequate manpower investment. ■
13 Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology
Glucose measurement Capillary Interstitial Interstitial Con rmation of accuracy Quality control check Calibration twice daily Not required Finger prick required Yes Yes to calibrate If display indicates hypoglycaemia Duration of strip/sensor Single use Six-to-seven days 14 days Data updating Manual update Every ve minutes to device or pumpRequires scanning using reader Alarm setting No Yes No Visibility Finger prick Disposable sensor is worn on the abdomen Disposable sensor worn on the back of the arm
HOME BLOOD GLUCOSE METERS (HBGM)CONTINUOUS GLUCOSE MONITORING (CGM)FLASH GLUCOSE MONITORING (FGM)
TABLE
1:
Methods for self-monitoring of blood glucose
Closed loop insulin delivery in type 1 diabetes mellitus
AUTHORS: Dr Christine Newman,1 Research Registrar; and Prof Fidelma Dunne,1,2, Consultant Endocrinologist
1 Department of Endocrinology and Diabetes Mellitus, Galway University Hospital
2 College of Medicine, Nursing and Health Sciences, National University of Ireland Galway
Type 1 diabetes mellitus (T1DM) is a complex, multi-system disorder with chronic health implications. To reduce potential microvascular complications, the American Diabetes Association (ADA) recommends a target haemoglobin A1c (HbA1c) of 53mmol/mol (7 per cent). Despite advances in diabetes technology this target is achieved by only 18 per cent of adults and 21 per cent of children (<18 years old) with T1DM.
In addition to suboptimal control, 7 per cent of all patients with T1DM will experience at least one episode of severe hypoglycaemia per year. Such admissions have significant emotional and economic impact. It is also well described that a fear of hypoglycaemia can lead to a reluctance to achieve good glycaemic control.
Advances in insulin delivery and real time glucose level monitoring have improved diabetes control in recent years. The use of continuous subcutaneous insulin infusion (CSII) can reduce both HbA1c and the frequency of hypoglycaemia when compared to multiple daily injections (MDI). Furthermore, real time continuous glucose monitoring (rt-CGM) can lead to improved glycaemic control and reduced rates of severe hypoglycaemia in those with T1DM on MDI. When combined with CSII, CGM can further improve HbA1c and time spent in hypoglycaemia.
Sensor augmented pump therapy, a further advance where insulin administration is automatically suspended at low glucose levels without the need for user intervention, can reduce severe hypoglycaemia in both paediatric and adult populations, however without
statistically significant impacts on HbA1c.
This compelling data combined with quality of life improvements seen with technology use have led to the ADA recommending CSII be considered for “all children and adolescents, especially children under seven years diagnosed with T1DM. Internationally, 44.4 per cent of children with T1DM use CSII therapy.
Though insulin pump therapy has been around in its earliest form since the 1970s, and sensor-augmented pump therapy was undoubtedly a great advance for patients with diabetes, diabetes technology took a further step forward in 2016 when the US FDA approved the first closed loop insulin delivery system for adults. Closed loop insulin delivery continuously feeds real time glucose readings back to the individual’s pump, and automatically makes adjustments without the need for the wearer to change their settings; in this way it is superior to sensor augmented pump therapy.
This article will explore the recent evidence behind closed loop technology and its potential advantages.
Closed loop published data
The ‘Six-month randomised, multicentre trial of closed loop control in type 1 diabetes’ and the ‘Randomised trial of closed-loop control in children with type 1 diabetes’ studies evaluated the e ectiveness of a closed loop insulin delivery system in reducing hypoglycaemia and improving time in range in patients aged 14 years and above and six-13 years respectively. Both of these studies were the longest of their kind published to date.
Both trials were funded by Tandem Diabetes Care and the National Institute of Diabetes and Digestive Kidney Diseases.
Study design – adult study
This was an open-label, multicentre, randomised, controlled study for patients with T1DM aged 14 years or older. Participants were recruited from seven centres in the US. A total of 168 patients were randomised in a 2:1 ratio to receive either a closed loop insulin delivery system (t:slim X2 insulin pump and Dexcom G6 sensor connected by ControlIQ Technology – an algorithm developed in the University of Virginia, US) or a sensor augmented pump (Dexcom G6 with the patient’s personal pump). As the t:slim had not yet received FDA approval an investigational device exemption was granted by the FDA.
All patients had T1DM and had been on insulin therapy, either CSII or MDI, for at least a year prior to randomisation. After enrolment all patients received a two-week run in to optimise glycaemic control. The run-in period was extended up to eight weeks for those unfamiliar with the technology. The patients had site visits at two, six, 13 and 26 weeks and had phone consultations in between these visits. They were required to upload data from their device before each visit and regularly during the trial. HbA1c was also checked twice during the study- at baseline and at 13 and 26 weeks.
This study was briefly interrupted for 33 patients due to a software error in the Control IQ algorithm. Data was included during this period as the patients continued to use the devices in ‘open loop’
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 14
mode ie glucose levels were received by the user and not delivered directly to the pump. The patients then made manual adjustments to their pump settings.
The primary outcome was the percentage of time patients spent with a glucose level of 3.9-10mmol/L. The secondary outcomes included time spent >10mmol/L and 13.9mmol/L, and below 3.9mmol/L and 3mmol/L, average glucose levels, HbA1c at 26 weeks, admissions for DKA and severe hypoglycaemia.
Results
In total 168 patients were randomised between July and October 2018, with 112 patients included in the closed loop group and 56 in the control/SAP group. The average age of participants was 33 years (range 14-71). Most patients had a BMI of >25kg/m; and patients were well matched in both groups for baseline HbA1c (7.6 per cent), educational and financial income status, previous CGM and CSII use. There was excellent retention with a 0 per cent drop-out rate and 100 per cent and 99.9 per cent of inperson and virtual visits completed.
Primary outcomes
The primary outcome of this trial was time in range (3.9-10 mmol/L) and this was achieved in 71 + 12 per cent (increased from 61 + 17 per cent) in the closed loop group and remained unchanged at 59 + 14 per cent in the control group. The change in time in range was statistically significant at 11 percentage points (95 per cent CI 9-14, p<0.001). These changes were seen within one month of therapy and continued for the duration of the trial.
The greatest discrepancy in glucose levels between closed loop and SAP was in the early morning between 5-6am.
Secondary outcomes
For these outcomes, closed loop technology was significant better than SAP therapy for percentage of time with glucose above 10mmo/L and below 3.9 mmol/L and average glucose levels. There was no change in HbA1c or hypoglycaemia < 3mmol/L. Rates of hypoglycaemia were improved in the SAP group at six months compared to baseline, however when reviewing the hypoglycaemia data in the control group it is extremely important to note that the pumps in the control group did not suspend insulin delivery for predicted hypoglycaemia.
There was a very high rate of device compliance use over the trial period; patients wore their devices for 97 per cent of the time (interquartile range, 96 to 98) in the closed-loop group and 96 per cent of the time (interquartile range, 90 to 97) in the control group. In the intervention group, patients used the closed loop mode 90 per cent of the time (interquartile range, 86 to 94).
The patients were also followed for adverse events. In total, 17 adverse events were reported in the closed loop group and 2 in the control group. There was one incidence of DKA (closed loop group); and there was a statistically significant difference in the rate of median hyperglycaemic events per week in the control, group (2.7 vs 1.2; p<0.001)
Study design – paediatric study
This was an open-label, multicentre, randomised, controlled study and included two parallel trials for patients with T1DM aged between six-13 years. Participants were recruited from four centres in the US. A total of 101 patients were randomised in a 3:1 ratio to receive either a closed loop insulin delivery or a sensor augmented pump.
All patients had T1DM for at least one year prior to initiation of the trial and had been on insulin therapy for at least six months. Participants were using at least 10 units of insulin as a total daily dose. Only those with a body weight of 25-140kg were included.
After enrolment any patient who was unfamiliar with CSII or CGM was given a 14-day run in period during which time they had to wear the devices for at least 11 out of 14 days. Fifteen out of the 23 patients (62 per cent) were given a CSII which suspended insulin delivery at a predicted low glucose level. Adjustments to CSII settings to maximise glycaemic control were made at the discretion of the investigator.
The patients had site visits at two, eight, and 16 weeks and had phone consultations in between these visits. They were required to upload data from their device before each visit and regularly during the trial. HbA1c was also done twice during the study at baseline and at 16 weeks. The primary and secondary outcomes were the same as the previous trial.
Results
In total 101 patients were included in the study, which was conducted between June and August 2019, of which 78 were assigned to the closed loop and 23 to the control/sensor augmented pump group. The mean age was 11.3 + 2.0 years in the closed loop group and 10.8 + 2.4 years in the control group. Average duration of disease was five and six years in closed loop and control groups respectively. Body mass index, starting HbA1c and insulin dose per kg, sex, ethnicity, parental education level were all included and comparable across both groups. A discrepancy was detected in annual household income in individual indices, however, overall there was a balance across both groups.
The majority of patients were familiar with CSII and CGM technology prior to the trial and only one patient in the control group dropped out of the study. There was >90 per cent attendance at both in-person and virtual meetings and 19 unscheduled visits in the closed loop group.
The frequency of study device use was comparable in both groups (median 97 per cent over 16 weeks [IQR 95-98] in the closed loop group) and 93 per cent (IQ
15 Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology
DAYTIME CL DAYTIME SAP NIGHT TIME CL NIGHT TIME SAP % OF TIME IN RANGE 70597659
range 91-95 per cent in the SAP group.
Primary outcomes
Percentage time in target range increased from 53+ 17 per cent to 67 + 10 per cent in the closed loop group and from 51 + 16 per cent to 55 + 13 per cent in the control group (p<0.001). This improvement was apparent very early in the trial and continued until week 16.
The closed loop group had persistently better rates of time in range both during the day and nocturnally , with a greater di erence observed during the house of midnight to 6am.
There was no DKA or severe hypoglycaemia in either group, however, the rate of hyperglycaemia and ketosis without DKA was 15 per cent in the closed loop vs 4 per cent in the control group.
Discussion
In these two associated multicentre, randomised controlled trials, children aged six-13 and adults aged 14 years upwards were randomised to closed loop insulin delivery or sensor augmented pump therapy. Both the adult and paediatric closed loop groups spent a significantly greater amount of time within the target range of 3.9-10mmol/L. The use of a closed loop system culminated in an additional 2.6 hours per day spent in the target range for both paediatric and adult populations. This was accompanied by a non-significant reduction in HbA1c compared to SAP therapy for both trial populations.
and there were very high rates of CSII and CGM use prior to trial entry.
In the adult cohort 34 per cent of patients in each group had a HbA1c <53mmol/mol and the average HbA1c was 60mmol/mol. Seventy per cent of patients used either CSII or CGM at baseline – the average use of CSII in Ireland in adult patients is 10 per cent.
While these trials establish the superiority of closed loop therapy, cost-e ectiveness will have to be clearly demonstrated before they can enter common clinical usage.
The improvements in time in range observed in the closed loop group are independent of age, sex, baseline BMI, HbA1c or previous device use.
Secondary outcomes
Improvements in HbA1c levels were greatest in those with a baseline A1c of >8 per cent (64mmol/mol), those with lower rates of hypoglycaemia, those with a greater rate of glycaemia >10mmol/L and in patients who had not previously been using this technology. Within this subgroup the changes were greatest in those who used neither CGM or CSII > CSII only > CGM only > pump+CGM.
Statistically significant changes were also observed in time spent above 10mmol/L in the control group and mean glucose level (9mmol/L in the closed loop group vs 9.9mmol/L in the control group).
The final HbA1c between the two groups did not meet statistical significance, however, the percentage of patients who met the target of 53mmol/mol rose from 28to-51 per cent in the closed loop group and from 13-to-18 per cent in the control group.
The adult group also benefitted from a reduced rate of hypoglycaemia (<4mmol/L) but not hypoglycaemia to level <3mmol/L. Within the paediatric population there was a slight increase in the number of hypoglycaemic events during the trial in both the closed loop and controlled groups but this did not reach statistical significance.
Both trials demonstrated an improvement in glycaemic control within the first month which persisted for the duration of the trial.
These studies are the largest and longest studies done in closed loop insulin delivery to date, with previous trials being 12 weeks in duration. Other trials evaluating closed loop systems vs SAP have demonstrated similar improvements in time in range and HbA1c.
While displaying impressive results these trial populations were not necessarily reflective of the general T1DM population.
The paediatric trial had a higher than average rate of HbA1c control (28 per cent of patients in the closed loop ground had a HbA1c of <53 mmol/mol at recruitment)
Currently there is no published data directly comparing the cost e ectiveness of closed loop therapy versus SAP. A 2018 Swedish study compared closed loop therapy to CSII alone (without CGM) and proved closed loop therapy to be cost e ective. The population in this study had an average HbA1c of 57mmol/ mol (7.4 per cent). Closed loop therapy reduced the rates of all microvascular and macrovascular complications at a cost of €16,000 per quality-adjusted life year (QALY) gained. A sub analysis of this study found the cost per QALY was less in patients with poorly controlled disease at baseline. A further study in a UK population found a cost of £20,000 GBP per one QALY. A 2015 French study evaluating the cost e ectiveness of treatment with a sensor augmented pump with low glucose suspend versus CSII alone found a cost of €30,163 per QALY in patients with high glycosylated haemoglobins. In patients at high risk for hypoglycaemia the cost per QALY was reduced to €22,005.
These studies contribute significantly to clinical knowledge in the field of diabetes technology, however based on the current low use of CSII and CGM in Ireland and the state support of flash glucose monitoring for patients aged four-21 years, it will likely be some time before they are in routine clinical usage. ■
References on request
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 16
DAYTIME CL DAYTIME SAP NIGHT TIME CL NIGHT TIME SAP % OF TIME IN RANGE 63568054
LET US HELP YOU MANAGE DIABETES
Blood Glucose monitoring System (IDEAL FOR TYPE 1 & 2 PATIENTS).


Suitable for Gestational
Suitable for gestational patients³





SYSTEM ACCURACY
99.33% of 4SURE SMART DUO test results are within ± 0.83 mmol/L (low glucose levels) or ± 15% (high glucose levels) of laboratory reference method test results. 4





TO ORDER YOUR FREE METER CONTACT: 1800 26 26 26





1. Haematocrit range 0-70% (blood glucose), 10-70% ( -ketone)


2. For driving with diabetes, follow DVLA guidelines






3. Follow NICE guidelines “Diabetes in pregnancy: management from preconception to the postnatal period” 4.
Clonmel Healthcare Ltd., Clonmel, Co. Tipperary. Always read the instructions before use. Date prepared: October 2019. 2019/ADV/4SU/113H.
SMART

Blood Glucose & ß-Ketone monitoring System (IDEAL FOR TYPE 1 PATIENTS).


















Ketone


Affordable blood glucose and -ketone test strips









Assessment of System Accuracy, Intermediate Measurement Precision, and Measurement Repeatability of a Blood Glucose Monitoring System Based on ISO 15197. Jendrike N, et al., J Diabetes Sci Technol. 2018 Dec 14.
β
Meal Pre AC Post PC 0-70% HCT Suitable for drivers Pre and post meal markers
for drivers² Wide haematocrit range¹ Bluetooth connectivity
Suitable
Additional Feature
Additional Feature
DIABETES CARE
The latest treatment approaches in diabetes
AUTHOR: Eamonn Brady, MPSI, owner of Whelehans Pharmacies, Mullingar
The Diabetes Federation of Ireland estimates that there are over 200,000 diabetics in Ireland and that over half of these have no idea they have diabetes.
The aim of diabetes treatment is to do what the body once did automatically, which is to mimic the insulin pattern before diabetes and to keep blood sugar under control.
For type 1 diabetes, insulin is always part of treatment as the body does not produce any insulin. In type 2 diabetes, the body still produces some insulin, so non-drug options or medication may be used to help the body make better use of the insulin that it still produces.
Treatment of type 1 diabetes
There is no cure for type 1 diabetes, but it can be kept under control. Type 1 diabetes is controlled by administering insulin. This allows glucose to be absorbed into cells and converted into energy, stopping it building up in the blood.
Treatment of type 2 diabetes
Many with type 2 diabetes can manage to control their condition simply by changing their lifestyle. Changes include:
Diet
A healthy diet is essential and it is important to eat regularly, three times a day. Special diabetic foods are not needed for a healthy diet; eating a balanced diet that is low in saturated fat, sugar and salt and high in fibre, vegetables and fruit is su cient. Include carbohydrates such as pasta, potatoes or sugary foods such as fruit in each meal.
There is strong evidence that progression from hyperglycaemia to type 2 diabetes can be prevented or at least delayed by dietary e ort. A diabetes prevention programme in the US recorded a 58 per cent reduction in the incidence of diabetes when participants
were provided with lifestyle intervention, including diet, compared with a 31 per cent reduction in persons treated with metformin.
However, there are relatively few other studies evaluating the effect of dietary intervention in patients with type 2 diabetes.
Exercise
Exercise promotes a healthy circulation and maintains a healthy weight. At least half an hour of moderate activity on at least five days a week is recommended. A Cochrane review showed that exercise significantly improved glycaemic control and reduced plasma triglycerides and visceral adipose tissue, even without weight loss. A high levelof leisure-type physical activity has been associated with a 33 per cent drop in fatal cardiovascular disease, while moderate activity showed a 17 per cent drop compared with the most sedentary group.
Smoking
It is especially important for diabetics to quit smoking. This is because a diabetic
CAUSES AND RISK FACTORS
Type 1 diabetes
Type 1 diabetes develops when the insulin-producing cells in the pancreas have been destroyed. It is not known for sure why these cells have been damaged but the most likely cause is an abnormal reaction of the body to the cells. This may be triggered by a viral or other infection.
Type 2 diabetes
Age
Risk of diabetes increases in patients aged over 40 or over 25 in African and Asian patients.
already has a five-fold increased chance of developing cardiovascular disease or circulatory problems compared to nondiabetics. Smoking makes the chances of developing these diseases even greater.
Alcohol
There is no need to give up alcohol completely, but it is important to drink in moderation.
Do not drink on an empty stomach. Eat foods containing carbohydrates before and after drinking (as alcohol reduces glucose levels). Monitor blood glucose levels more regularly if drinking alcohol.
Medication
If lifestyle changes alone do not reduce glucose levels, medication may be used to increase insulin production and strengthen its e ect. All of the types of oral hypoglycaemic drugs and insulin are safe in older patients, although each has some limitations in older people. ‘Start low and go slow’ is a good principle to follow when starting any new medications in an older adult.
Family and ethnicity
Having diabetes in the family increases the risk of diabetes. The closer the relative is, the greater the risk. People of Afro-Caribbean or South Asian origin are at least ve times more likely to develop diabetes.
Weight
Over 80 per cent of people diagnosed with type 2 diabetes are overweight. The more overweight and inactive the person is, the greater their risk of diabetes.
Waist size
Women: If the waist measures 31.5in (80cm) or more, the risk of diabetes increases.
Men: If the waist is 37in (94cm) or more, this means increased risk of developing diabetes.
Gestational diabetes
Pregnant women can develop a temporary type of diabetes called gestational diabetes. Having this, or giving birth to a large baby, can increase the risk of a woman going on to develop diabetes in the future.
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 18
Metformin improves the e ectiveness of insulin by reducing the amount of glucose released from the liver and improving the way glucose is used by muscles. It causes less weight gain than other diabetic medication.
In the UKPDS, metformin reduced diabetesassociated deaths by 43 per cent over a 10-year follow-up. Metformin acts on the liver to lower production of glucose and reduce glycogenolysis; it has no e ect on insulin release so is not associated with hypoglycaemia. Up to 3g of metformin can be administered daily. A 2005 Cochrane review concluded that no other antidiabetic drug showed more benefit in terms of glycaemia control, body weight or lipids than metformin in type 2 diabetes. Gastrointestinal (GI) upset is the most common side-e ect and has been reported in up to 50 per cent of patients. Metformin is an attractive agent to use in the elderly due to a low risk of hypoglycaemia. However, it should be given with caution in older diabetic patients because of the risk of lactic acidosis. Older patients often have impaired renal function despite an apparently normal serum creatinine concentration. Weight loss and GI side-e ects may also be limiting factors in older adults taking metformin. Therefore, metformin should be used with caution in older patients.
Sulphonylureas encourage the pancreas to produce more insulin. Because they stimulate insulin secretion, they are most e ective in newly- or recentlydiagnosed patients who still have relatively active beta cell function. In the UKPDS, sulphonylureas reduced diabetic-related deaths by 36 per cent.
Sulphonylurea monotherapy is e ective in 75-to-80 per cent of patients with type 2 diabetes; however treatment
‘failure’ occurs at a rate of 5-to-10 per cent of patients per annum. Sulfonylureas are usually well tolerated in older patients; however hypoglycaemia is the most common side-e ect and more common with older, long-acting sulfonylurea drugs. However, gliclazide MR is well tolerated in elderly patients.
Thiazolidinediones reduce the body’s resistance to insulin and are generally limited to use with metformin and sulphonylureas if other standard treatments were not working or not tolerated. They are licensed as monotherapy in obese patients if metformin is ine ective or not tolerated. They work by reducing insulin resistance in adipose tissue, muscle and liver. They take up to 12 weeks to exert their full a ect. Thiazolidinediones are useful for some older diabetic patients because they can be given to patients who have impaired renal function. They are well tolerated in older adults and do not cause hypoglycaemia. However, limited experience, high cost and concerns regarding fluid retention, congestive heart failure, myocardial infarction (MI), and fractures limit their usefulness. Weight gain is a side-e ect so they should be avoided in obese patients.
Rosiglitazone was withdrawn from the European market in October 2010 because research showed it increased the risk of cardiovascular problems, including increasing the risk of MI and heart failure. It was considered that its benefits no longer outweighed its risks. Pioglitazone is still on the Irish market, but must be used with caution in those with cardiovascular problems.
Newer medicines such as DPP-4 inhibitors, (sitagliptin) help the body to produce more insulin in response to meals. They work by enhancing the levels of active incretin hormones, thus enhancing insulin and reducing glucagon secretions.
DPP-4 inhibitors do not cause weight gain and only rarely cause hypos.
The combination of sitagliptin and metformin has been shown to be better tolerated (14.5 per cent incidence of adverse e ects) compared with a combination of sulfonylurea and metformin (30.3 per cent incidence of adverse e ects). Long-term safety with this class of drug in the elderly has not been established.
Acarbose lowers blood glucose by slowing the breakdown of some carbohydrates.
Acarbose reduces the digestion and absorption of starch and sucrose by competitively inhibiting the intestinal enzymes involved in the degradation of disaccharides, oligosaccharides and polysaccharides.
The reduction in HbA1c is modest, however it is very unlikely to cause weight gain. The main side-e ects, which a ect over 10 per cent of patients and limit its use, are flatulence and diarrhoea. The GI side-e ects are due to intracolonic fermentation of the unabsorbed sugars and reduce with time. Acarbose has not been widely tested in elderly diabetic patients, but is likely to be fairly safe and e ective.
Meglitinides include repaglinide and nateglinide. Meglitinides act as postprandial glucose regulators. They increase insulin secretion by binding to specific sites within the ß cells in the pancreas. They have a rapid onset of action, meaning they need to be administered shortly (one-to-30 minutes) before meals. They have a short duration of action, requiring multiple daily dosing. Long-term data on treatment outcomes of meglitinides are not yet available. Meglitinides may play a useful role in patients with irregular meal times (ie, shift workers). GI upset is generally less than with metformin, however weight gain is greater than experienced with metformin and can be as much as 3kg in three months. Because they are metabolised by the cytochrome P450, their potential for interactions is greater than other oral anti-diabetics. Their use is limited by many factors, including their inconvenient dosage regimen, ie, they must be taken immediately before meals and need multiple daily dosage. Other oral anti-diabetics have more convenient dosage regimens.
Exenatide is a synthetic peptide drug that works by increasing the secretion of insulin and reducing secretion of glucagon in response to hyperglycaemia. It is administered by subcutaneous injection twice daily.
The two drugs available in this class in Ireland (and allowed on the GMS) are Bydureon and Byetta. GI upset (including
19 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
nausea, vomiting and diarrhoea) was recorded in up to 50 per cent of patients in clinical trials. Hypoglycaemia occurred in over 10 per cent of patients when combined with a sulfonylurea in clinical trials, so the dose of the sulfonylurea should be reduced if combined with exenatide. Further postmarketing safety and long-term outcome data is required for this drug.
Fast-acting insulin preparations include insulin aspart and insulin lispro. The onset of action of insulin aspart is 10-to-20 minutes and the duration of action is threeto-five hours.
Insulin lispro has an onset of action of approximately 15 minutes and duration of action of two-to-five hours. Insulin aspart is injected subcutaneously immediately before meals or, when necessary, soon after. Insulin lispro is injected subcutaneously shortly before meals or, when necessary, soon after. Insulin aspart and insulin lispro are normally used in combination with longeracting insulin to provide a steady glucose level throughout the day. The dose of insulin aspart or lispro needs to be individualised to the patient. The fast-acting insulins may also be used for continuous subcutaneous infusion using an insulin infusion pump.
Biphasic preparations are also available. These contain fast-acting (aspart or lispro) and intermediate-acting insulin analogues. The long-acting insulin glargine is administered subcutaneously once daily. It can be administered at any time, but it should be given at the same time each day. The dosage and timing of administration should be individualised to the patient. It shows a constant concentratione ect versus time profile which lasts approximately 24 hours. Insulin glargine is used as an additional therapy to oral hypoglycaemic agents in type 2 diabetes where oral hypoglycaemic agents do not provide adequate glucose control on their own. Insulin glargine is also associated with similar or less weight gain compared with isophane insulin (intermediate action).
Patients with both type 1 and 2 diabetes experience greater diabetic control with
insulin glargine compared with isophane insulin, studies show.
Interactions with other drugs must be taken into account when determining dose of insulin. Drugs which reduce hypoglycaemic activity and may increase insulin requirement include oral contraceptives, thiazides, glucocorticoids, thyroid hormones, sympathomimetics and danazol. Substances that enhance hypoglycaemic activity and may decrease insulin requirements include oral anti-diabetics, certain antidepressants (MAOIs and SSRIs), non-cardio selective beta blockers, certain ACE inhibitors (captopril, enalapril), angiotensin 2 inhibitors, aspirin, alcohol, anabolic steroids, and sulphonamides.
Beta blockers also mask the symptoms of hypoglycaemia and alcohol may intensify and prolong the hypoglycaemic e ect of insulin.
Insulin is sometimes underutilised in the elderly because of fear (by the doctor, patient, or family) that it is too complicated or dangerous. With the availability of longacting insulins, ie, detemir, glargine, it has become easier to add once-daily insulin to oral hypoglycaemic medications in older patients who have poor glycaemic control.
DIABETES SYMPTOMS
▸ Frequent urination
▸ Excessive thirst
▸ Extreme hunger
▸ Increased fatigue
▸ Irritability
▸ Increased weight loss
▸ Blurred vision
▸ Genital itching or regular thrush
▸ Slow healing of wounds
Type 1 diabetes symptoms develop very quickly, usually over a few weeks.
In people with type 2 diabetes, the signs and symptoms will not be so obvious or may even be non-existent. If a person is older, they may put the symptoms down to ageing.
The quality-of-life of many older patients improves substantially when they take one or two daily doses of intermediate- or longacting insulin.
Which drug to choose?
Step1
▸ Metformin remains the most e ective monotherapy, especially in obese patients.
▸ Sulfonylureas remain a good choice if the patient is not overweight.
▸ Repaglinide and acarbose are also authorised for monotherapy, but are rarely used due to reasons described above.
▸ Thiazolidinediones may be used as monotherapy only in overweight patients if metformin is ine ective or not tolerated but should be avoided in patients with cardiovascular problems.
Step 2
If monotherapy is ine ective, Step 2 is dual therapy, which includes metformin and/ or sulfonylurea and/or thiazolidinedione. Sitagliptin is a popular add-on therapy to metformin due to its success and low incidence of side-e ects. Meglitinides, acarbose and exenatide are also authorised for combination therapy.
Step 3
Step 3 is triple-therapy (metformin and/or sulfonylurea and/or thiazolidinedione and/ or meglitinides and/or acarbose). Insulin therapy may be considered with dual therapy but should be initiated by a specialist.
It must be borne in mind that metformin and sulphonylureas provide a greater reduction in HbA1c than any of the newer oral antidiabetic drugs. Metformin and sulphonylureas reduce HbA1c by greater than 1.5 per cent; no other oral antidiabetic drug reduces HbA1c by greater than 1.5 per cent. Metformin and sulphonylureas are central to any treatment regimen of type 2 diabetes. Metformin and sulphonylureas are also less expensive than other oral antidiabetic drugs. ■
References on request
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 20
Diabetes and the new HSE chronic disease management programme
AUTHOR: Dr Diarmuid Quinlan, Cork GP and author of the Diagnosis and Management of uncomplicated Type 2 Diabetes in Adults, (T2DM) ICGP guide (2019)
“The way we currently care for (people with) chronic conditions is relatively ine ective, ine cient and ultimately unsustainable. Too many people end up needing hospital admission… too many depend on hospital OPD. We need a better way of caring for people with chronic diseases… a ecting approx. 1 million people.”
In this article I will outline the new chronic disease management (CDM) programme recently launched by the HSE, and focus on management of type 2 diabetes mellitus (T2DM) within this programme. I will especially focus on our clinical role in the foot assessment of people with T2DM.
There are approximately one million people in Ireland with one or more chronic diseases. These people should receive care of high quality that is readily accessible and at modest cost to our health service. We clinicians in general practice deliver these core principles of quality, access, and cost.
The CDM programme is a most welcome initiative, supporting high-quality management of diabetes, COPD, asthma, and cardiovascular diseases. Many people have three or more chronic diseases, presenting clinical challenges in older patients. The current unstructured care pathways are “relatively ine ective, ine cient, and ultimately unsustainable”. The CDM programme should modernise our care for these patients.
Clinical reviews
The CDM programme supports two structured clinical reviews per year. These will most commonly include both practice nurse and GP. The programme is limited to people with medical cards, commencing with patients aged 75 years of age and over, with phased expansion to include all adults with GMS
cards by 2023. Many of us already undertake this CDM work. We need to implement e cient workflows and avoid duplication. We can deliver the right care, at the right time to the right patient. The practice nurse visit will usually include ‘talking (patient education), tests and tasks’.
Talking: Patient education is an integral part of managing chronic disease. Practice nurses routinely address the key lifestyle issues of smoking, exercise, alcohol, weight/BMI. Many clinicians will benefit from support around how best to raise and address these issues in a sensitive fashion.
Tests: There are no ‘routine bloods’ and we should not tick every box on the laboratory form: Excessive blood testing is a poor use of costly laboratory resources and increases the work of reviewing results and patient follow up. The GP software systems will prompt which blood tests are required. It is important to document height, weight, BP, and waist circumference in the appropriate data field in your IT system to enable auto-population. Expect some teething problems.
Many GP practices are upskilling a phlebotomist, releasing clinician time for more complex patient care. Schedule the blood test so results are available for the CDM review with the GP.
Tasks will include, pulse, BP, weight, height, waist circumference, influenza, and, where appropriate, pneumococcal vaccine, 24-hour ABPM, ECG, and possibly referral to dietetics/ retina-screen/podiatry, etc, as appropriate. Some nurses with appropriate training and equipment (128Hz tuning fork, 10g monofilament) may undertake the annual foot assessment (see below).
The patient care plan is a new concept,
formalising sharing information with patients. The care plan shares information and engages our patients in their healthcare. There is an evidence-base underlying the patient held care plan, identifying and incorporating our patient preferences, priorities and goals. The computer system will ‘auto-populate’ much of the patient care plan. The care plan is an important aspect of supporting our older, complex, multimorbid, and increasingly frail patients to identify and prioritise their personal goals.
The GP visit will include:
▸ Review, management and follow-up of investigation results.
▸ Clinical review of the patient.
▸ Patient education.
▸ Medication review and optimisation.
The CDM programme will support general practice to deliver high quality care to our older patients. In summary: Each GMS patient over 75 years (and all GMS adults by 2023) will have two such visits each year. There will be appropriate blood testing followed by a clinical assessment.
Key objectives and targets in T2DM
▸ Early and accurate diagnosis of all people with T2DM.
▸ Weight loss 5-10 per cent (or >10kg), document waist circumference.
▸ Target HbA1c </= 58, ideally <53.
▸ Target BP 120-139/70-79.
▸ Lifestyle interventions: Document exercise, smoking, alcohol.
▸ Recommend a statin if ≥40 years, target LDL cholesterol <2.
▸ Foot care: Examine regularly and refer if abnormal (foot care is often neglected).
▸ Flu and pneumococcal vaccine, audit to drive quality improvement.
▸ Retinopathy: Refer and attend diabetic retinopathy screening programme, audit
21 Endocrinology and Diabetology | Volume 8 | Issue 6 | 2020
your practice?
▸ Think kidneys: Check ACR and eGFR.
▸ Mental health matters.
Eyesight
Diabetes is the leading cause of adult blindness (excluding trauma). The national diabetic retinopathy screening programme, RetinaScreen, has made substantial progress in recruiting people. There are still many eligible people not availing of retinopathy screening, however. Please check if your patients are registered and attending.
Diabetes and foot disease
We have made very substantial progress in reducing the number of people with T2DM having toe/foot amputations in Ireland. However, there remains scope for substantial improvement.
People with diabetes often develop peripheral sensory impairment. This sensory loss predisposes to tissue damage in the foot. Impaired circulation delays healing. The combination of ischaemia with sensory loss cause a downward spiral of skin damage, ulcers, infection, osteomyelitis, gangrene, and amputation.
Foot ulcers are harbingers of impending amputation. Most ulcers can be prevented with good foot care and foot screening.
Key messages in diabetic foot disease
▸ Foot ulcers a ect 10 per cent of people with diabetes and almost half die within five years of developing a foot ulcer. Most ulcers can be prevented with good foot care.
▸ Examine foot at diagnosis and at least yearly: Inspection, sensory, and vascular assessment.
▸ Loss of vibration sense at the big toe is a very early sign of neuropathy: Refer to podiatry.
▸ Stratify into normal and abnormal feet; refer people with ‘abnormal’ feet.
▸ Most amputations start with foot ulcers. Refer people with foot ulcers or foot infection to hospital immediately or next working day.
▸ Provide foot-care education to all patients with T2DM.
The foot in diabetes: Examine, stratify, protect Foot examination: The three components to a systematic concise foot assessment are:
Inspection: Assess skin, nails, bones and footwear: check for macerated web spaces, ulcers, hair loss, deformed toenails and foot deformity.
Sensory assessment: This has two components:
▸ 10g monofilament: Test at between fourto-10 sites on each foot.
▸ Vibration sense: use a 128Hz tuning fork at tip of big toe. Can your patient identify the vibration, and cessation of vibration? Absent sensation suggests sensory neuropathy.
Circulation assessment: Assess capillary refill time, posterior tibial and dorsalis pedis pulses, oedema, skin temperature, oedema etc. This is often quite di cult, even for experienced clinicians. Your local podiatrists are a fantastic resource for training.
The American Academy of Physician Assistants has an excellent diabetic foot examination 10-minute training video (www. youtube.com/watch?v=GemhbvnoR6w).
Stratify the foot in T2DM
A very simple binary approach stratifies the foot examination into ‘normal’ or ‘abnormal”. People with any foot abnormality should be referred to the HSE podiatry service.
Normal examination: A patient with no history of ulcers, normal skin-nails-bones, normal foot pulses (vascular assessment), and no sensory loss. These patients are managed in general practice, with patient education and an annual foot examination.
Abnormal foot examination: Patients with any abnormality should be referred, ideally to HSE podiatry services. Foot ulcers or infection are an emergency and should be referred to hospital immediately or next working day. Ask the GP to make these emergency referrals.
The referral pathway will be influenced by local podiatry service provision. It is regrettable that many areas are deficient in podiatry services.
Protect
Patient foot care education in T2DM
Some practices use a ‘note template’ to document foot education in T2DM: ‘Address nail care, emollient use to dry skin, but not between toes. Encourage daily self-examination of the feet, never walk in bare feet (including beach), check footwear and hosiery before wearing, never ‘break shoes in’. Never use hot water bottles, always check bath and shower temperature with hand and not foot. Avoid home remedies, eg, corn plasters. Contact GP if any injury, ulcer, swelling, warmth, redness or pain in your legs or feet. The online HSE foot care leaflets are excellent.
Summary and conclusion
The CDM programme is a great initiative to support general practice in delivering high quality care to our patients. Many of our patients have multiple chronic diseases and we are skilled in managing these patients. The CDM programme is evidence-based, promotes high quality care, and many of us are already doing this work. The practice nurses currently undertake patient education, phlebotomy, and a host of other tasks when providing patient care.
Many practice nurses are skilled in the management of people with T2DM. This article touched on the key targets, then drilled down into foot care in people with T2DM. Assessment of the foot in diabetes involves a clinical examination of the foot, with sensory and vascular assessment. We divide the outcome into either ‘normal’ or ‘abnormal’. People with any abnormal finding on foot assessment should be referred, usually to HSE podiatry. Education of our patients around foot care is fundament to supporting our patients and protecting their feet.
The ICGP recently published Diagnosis and management of uncomplicated T2DM: A succinct practical guide for Irish general practice. This is readily available on the ICGP website, www.icgp.ie ■
References on request
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 22
doctorcpd.ie
Latest module
Type 2 diabetes and insulin initiation was devised by Dr Caoimhe Casey and Dr Ronan Canavan, Consultant Endocrinologist, St Columcille’s Hospital and St Vincent’s University Hospital, Dublin.

The main risks with insulin therapy are DKA and hypoglycaemia. True or False?
Check your answer against the latest module on doctorcpd.ie. Successful completion of this module will earn you 2 CPD credits
now accessible on android, iPhone and tablet
Free CPD –
A B C
Free independent CPD for Irish doctors by Irish doctors
Changing the landscape for delivery of type 2 diabetes care
AUTHOR: Dr Fiona Riordan, Postdoctoral Researcher, School of Public Health, University College Cork, Aut Even Hospital Kilkenny, and Senior Lecturer in University of Limerick Medical School
Diabetes has been described by the International Diabetes Federation as one of the greatest challenges of the 21st century. The rising number of people with diabetes, the chronic nature of the condition and associated complications places a burden on health systems. Internationally there has been a drive to move away from reactive, episodic management of type 2 diabetes in the acute setting to greater primary care-led structured disease management with the aim of delivering better quality care closer to home for patients. E orts to optimise care across Europe have seen the establishment of disease management programmes, which better organise management in primary care and aim to improve the co-ordination of care between di erent settings: the community, outpatient/ambulatory and inpatient settings.
Given that primary care provides firstcontact, continuous, comprehensive and coordinated care, it is a good place to start in terms of improving care. In Ireland, primary care management of diabetes is not standardised. Care delivery can vary regionally, from ad hoc and opportunistic management in primary care in some areas, to largely hospital-led management in others. E orts to better organise care delivery have included a number of national reforms,
including the establishment of the HSE National Clinical Programme for Diabetes, the Diabetes Cycle of Care, and publication of guidelines including A Practical Guide to Integrated Type 2 Diabetes Care (2016), which set out roles and responsibilities for the key professionals involved (GPs, diabetes nurse specialists, practice nurses) and targets for management; HbA1c, lipids, and blood pressure. (Table 1).
Development
A local need for improvements in the quality of care has driven a ‘ground up’ response, namely the development of primary carebased diabetes initiatives that better organise diabetes management in the community. In operation since 1997/1998, the HSE Midland Diabetes Structured Care Programme is the longest established such programme, led by Dr Velma Harkins, a GP Unit doctor, who was looking to utilise small funds from drug savings. She was inspired by the 1989 international St Vincent Declaration on Diabetes to use these funds to improve care of patients with diabetes in the area. In collaboration with Dr Davida de la Harpe, Specialist in Public Health, Dr Harkins conducted an audit of the care received by patients with diabetes in her own practice. A pilot of the structured programme was carried out in 1997 in the practice before
extending to 10 practices locally who had GPs with an interest in diabetes, enabling them to develop a partnership with the Department of Public Health and Planning in the then Midland Health Board. The programme was linked to the Cardiovascular Strategy in 2001 through the HSE Midland Area Primary Care Working Group and in 2002 the programme was integrated into the national cardiovascular disease secondary prevention programme in general practice: Heartwatch.
The programme is dedicated to improving the quality of care for patients with diabetes in the counties of Longford, Westmeath, Laois, and O aly, and comprises of several strategies to improve diabetes management, including the use of evidence-based clinical guidelines, patient register and recall and protected time for review visits, ongoing organisation and coordination of care by practice nurses, structured multidisciplinary support and professional and patient education. The programme comprises regular structured patient visits per year.
The initial assessment after diagnosis (first patient visit) includes the following elements:
▸ Diagnostic details such as patient name and address, type of diabetes, medical history, family history.
▸ Medical examination – weight, blood pressure, foot examination.
▸ Investigation – full blood count, liver function tests, ferritin, HbA1c.
▸ Referral of patient to various services such as dietitian, podiatry, retinal screening.
▸ Addition of the patient to the practice register and given a follow-up appointment. Regular review visits are arranged for the prevention, early detection and management of complications associated with type 2 diabetes.
These regular review visits include the following elements:
▸ Review of medications, hypoglycaemia/
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 24
TABLE
Clinical outcome Unit Target HbA1c mmol/mol (%)53 (≤7.0) Systolic blood pressure mmHg ≤140 Diastolic blood pressure mmHg <80 Total cholesterol mmol/l <4.5 Low-density lipoprotein (LDL) mmol/l ≤2.5 High-density lipoprotein (HDL) mmol/l ≥1.0 Triglycerides mmol/l ≤1.7
1: Clinical outcome targets according to Irish guidance published 2016
hyperglycaemia, dietary habits, physical activity.
▸ Medical examination – weight, blood pressure, foot examination.
▸ Investigations – HbA1c, urinary analysis.
▸ Referral follow-up.
Along with all of the areas monitored at regular reviews, surveillance of the following should also be carried out annually:
▸ Symptoms: Ischaemic heart disease, peripheral vascular disease - neuropathy, erectile dysfunction. All patients with symptoms that might reflect vascular disease, particularly ischaemic heart disease, should be investigated.
▸ Feet: Footwear, deformity/joint rigidity, poor skin condition, ischaemia, ulceration, absent pulses, sensory impairment.
▸ Eyes: Visual acuity and retinal review by ophthalmologist/retinal screening programme.
▸ Kidney: Renal damage, albumin excretion, serum creatinine and calculate eGF.
▸ Arterial risk: Blood glucose, blood pressure, blood lipids, and smoking status, ECG.

▸ Attendances: Podiatry/dietitian/ other as indicated.
The initial aims of the programme were to develop a model of care for people with diabetes suitable for the Irish healthcare environment based on the St Vincent Declaration and on best evidence, specifically:
▸ Raise the overall standard of care for people with diabetes in the region.
▸ Document the barriers to implementing the model of care.
▸ Develop methods to evaluate the e ect of changed processes on health outcomes for people with diabetes.
Expansion
Beginning with just 10 practices, the programme has continued to expand; by mid 2019 30 practices were participating in the programme and there were almost 4,000 patients enrolled (mid 2019). Over time, the programme has contributed to the evidence base for structured primary care-led diabetes management in Ireland, serving
as an example of what can be achieved through this approach to care delivery. The programme has brought structure and organisation to a previously ad hoc approach in primary care. As one participating GP reflected: “Before I joined the Midland Diabetic Structured Care Programme in 1999, the care that my patients with type 2 diabetes received was very disjointed and disorganised. As there was no consultant endocrinologist in the midlands, the patients with type 2 diabetes were being seen in the medical clinics in the hospital often only once a year.”
Since taking part in the programme practices have seen improvements, particularly in terms of the training and education facilitated through the programme: “Once the programme was initiated, my practice nurse and myself received specialist training in diabetes care. There were also guidelines written and we got access to a diabetes nurse specialist, specialist foot care and dietetics in the practice. Having access to the training and guidelines and feeling supported by the availability of the diabetes nurse specialist, gave me and my practice nurse the confidence to provide all the high-quality care these patients required in the practice.”
Recognition
A recent analysis of the programme demonstrated the sustainability of this model of care, with significant improvements in processes of care delivered to enrolled patients since first initiated in 1999 (Figure 1).
The programme underpins the model of care developed by the HSE National Diabetes
Clinical Programme, whereby management of patients with uncomplicated type 2 takes place in primary care. The programme is committed to ongoing monitoring and the outcomes from the programme have been communicated widely, through publicly available audit reports; the most recent of which was launched by the Minister for Health and received national media coverage.
In October 2018, the work of the Midlands programme was recognised as part of Quality in Care (QiC) Diabetes, an awards programme for the UK and Ireland that recognises good practice in patient care. The Midlands programme was a finalist for the Judge’s Special Award and received the certificate for highly commended. We were delighted to be chosen for this award. It has been a very exciting project from the start. It was a great privilege to be able to develop a model of care based in general practice and it has been very gratifying to see such excellent results achieved over the past 20 years. Its success is due to the excellent care provided by the practice nurse, CNS, dieticians, foot care specialists and by the support of local HSE primary care. Patients themselves have engaged with the programme with great enthusiasm, appreciating the ease of access to doctor appointments and the equity of care.
The project clearly demonstrates how a chronic disease can be managed in primary care when the appropriate resources are made available. We are looking forward to continuing to improve our care, and to continue to be involved with other research partners to help to best map outcomes and processes for this condition. ■
25 Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology
FIGURE 1: Patients with type 2 diabetes with care processes recorded 1999, 2003, 2009 and 2016
Osteoporosis: The silent disease
AUTHORS: Theresa Lowry Lehnen, PhD, RGN, Post Grad Coronary Care, BSc (Hons), Clinical Nurse Specialist Practitioner, Associate Lecturer at Institute of Technology Carlow, and Member of the Irish Student Health Association (ISHA)
Osteoporosis, commonly known as the ‘silent disease’, is characterised by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased risk of fractures. It is estimated that 300,000 people in Ireland and approximately 200 million people worldwide are a ected by the condition. Osteoporosis is the most common metabolic bone disease in Ireland and increases the risk of ‘fragility fractures’. These fractures occur mainly at the hip, vertebrae, and distal forearm and are associated with significant morbidity, mortality, and reduced quality-of-life, attributed not only to the fracture itself, but also to the high prevalence
of comorbidities in this patient population. The prevalence of osteoporosis is expected to increase significantly in the future because of population ageing. Osteoporosis is the most common bone disease worldwide and a major public health hazard, with high morbidity, mortality and socio-economic costs. Osteoporosis mainly occurs in postmenopausal women and elderly men. A postmenopausal woman’s annual risk of fracture is greater than her combined risk of cardiovascular disease and breast cancer. One-in-four men and one-in-two women over the age of 50 years will develop a fracture due to osteoporosis in their lifetime. The disease
SIGNS AND SYMPTOMS OF OSTEOPOROSIS
A fragility fracture occurs when a person sustains a broken bone from a force less than or equal to that sustained from a fall from a standing position. With severe osteoporosis even forces as minor as a cough, sneeze, turning over in bed or lifting a small weight can result in a fracture.
Development of a kyphosis - A stooped posture where the persons head is bent forward may result from an anterior wedge fractures of the spine. In severe cases a Dowager’s hump may develop on a person’s upper back, which is a strong indication that osteoporosis should be considered.
Loss in height 2-16cm. Height loss >2 inches is an important sign of an asymptomatic vertebral fracture and should be evaluated for osteoporosis. A person can lose height due to wear and tear of vertebrae and/or disc but >2 inches is unusual in degenerative joint and disc disease.
Change in body shape or size associated with loss of height. A distended abdomen can develop as the stomach and intestines push outwards followed by the rib cage resting on the pelvis. These changes can cause di culty in breathing, back pain, depression, loss of functional independence, and gastrointestinal symptoms.
Sharp sudden pain in the low, middle or upper back, especially with height loss should be evaluated for vertebral fractures as this may be the rst presentation of an osteoporotic fracture. When plain lms are normal and symptoms persist, repeat X-rays several weeks later or additional imaging may show a fracture. Causes of back pain should always be addressed and vertebral fractures ruled out.
can also a ect children.
According to the Irish Osteoporosis Society (IOS), 20 per cent of people aged 60 and above who sustain a hip fracture will die within six-to-12 months, due to secondary complications and 50 per cent of people over the age of 60 who sustain a fractured hip will lose their independence. Only 15 per cent of people in Ireland are actually diagnosed with bone loss, leaving an estimated 280,000 people undiagnosed.
Causes
Osteoporosis is multifactorial in origin. It occurs when there is an imbalance between new bone formation and old bone resorption. Bone turnover is regulated by the interaction between osteoblasts and osteoclasts. Osteoblasts form new bone and osteoclasts are responsible for bone resorption. Both types of cell are under hormonal regulation. Up to 90 per cent of peak bone mass is acquired by age 18 in females and age 20 in males. The amount of bone mass in the skeleton can keep increasing until a person reaches their late 20s, at which point, bones have reached their maximum strength and density, known as peak bone mass. As people age the rate of bone resorption by osteoclast cells exceeds the rate of bone formation, so bone weakens. The greatest cause of osteoporosis is oestrogen deficiency, which results in increased bone turnover in which resorption exceeds formation. Corticosteroids can also induce osteoporosis in which trabecular bone is particularly a ected from suppression of osteoblastic activity. Peak bone mass is the major determinant of adult bone density. Peak bone mass has a strong genetic component, with between 60-to-85 per cent of the variance in bone mineral density (BMD) being attributable to genetic factors. Certain risk factors are linked to the development of osteoporosis and contribute to an individual’s likelihood of developing
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 26
TABLE 1: Signs and symptoms of osteoporosis
Source: Irish Osteoporosis Society
MODIFIABLE RISK FACTORS
▸ Sex hormones: The reduction of oestrogen levels in women at menopause is a strong risk factor for developing osteoporosis. Men have a gradual reduction in testosterone levels as they age. Treatments for prostate cancer that reduce testosterone levels in men and treatments for breast cancer that reduce oestrogen levels in women accelerate bone loss.
▸ Endocrine: Too much thyroid hormone can cause bone loss. This can occur in hyperthyroidism or if too much thyroid hormone medication is used to treat an underactive thyroid. Osteoporosis has also been associated with overactive parathyroid and adrenal glands and hypogonadism.
▸ Medication use: Long-term use of certain medications, such as glucocorticoids and some anticonvulsants can lead to loss of bone density and fractures. Depo-Provera contraceptive has been proven to cause bone loss, which is particularly high risk if given during adolescence when bone is being laid down.
▸ Lifestyle factors: An inactive lifestyle can lead to weakened bones and increased risk of osteoporosis.
▸ Cigarette smoking and excessive consumption of alcohol increases the risk of bone loss and fractures.
▸ Poor diet increases the risk for osteoporosis. Low calcium and vitamin D intake contributes to diminished bone density, early bone loss and an increased risk of fractures.
▸ Eating disorders: Severely restricting food intake, low BMI and being underweight weakens bone in both men and women.
NON-MODIFIABLE RISK FACTORS
▸ Sex: Women are much more likely to develop osteoporosis than men. Women have less bone tissue and lose bone faster than men because of the changes that occur with menopause.
▸ Age: The risk of developing osteoporosis as bones become thinner and weaker increases with age.
▸ Body size: People who have small body frames tend to have a higher risk of developing osteoporosis because they have less bone mass to draw from as they age.
▸ Ethnicity: White and Asian women are at highest risk. African American and Hispanic women have a lower, but signi cant risk.
▸ Genetic: A family history of osteoporosis is a very strong risk factor. People whose parents have a history of fractures also seem to have reduced bone mass and may be at greater risk for fractures.
the disease. Some risk factors are modifiable while others are not.
Diagnosis
Osteoporosis is a silent disease without obvious symptoms until a fracture occurs. Bone turnover biomarker detection can be useful in monitoring osteoporosis treatment and assessing fracture risk but not for diagnosis of osteoporosis.
Osteoporosis can be diagnosed by:
1. The presence of a fragility fracture;
2. Measurement of BMD;
3. Bone biopsy.
Bone biopsy is restricted to untypical, unclear and complicated cases in evidence-based guidelines on diagnosis and treatment of osteoporosis. It is not routinely used and should never be undertaken without consultation with a specialist in osteoporosis and metabolic bone disease.
The majority of fractures occurring after 50 years of age are osteoporotic. All persons presenting with a fragility fracture after 50 or menopause should be considered as possibly osteoporotic. A detailed history of the fracture occurrence, physical examination and evaluation for other fractures is carried out while noting any presence of back pain, kyphosis, and height loss.
Baseline laboratory tests include: Full blood count; serum chemistry levels; liver function tests; thyroid-stimulating hormone level; 25-hydroxyvitamin D level; serum protein electrophoresis; 24-hour urine calcium/ creatinine; testosterone (total and/or free); and luteinising hormone/follicle-stimulating hormone. Additional testing should include measurement of BMD and if there is height loss and/or back pain, imaging of the spine. On average, BMD is lower in women than in men, because women have smaller bones and smaller trabeculae. Women, as they also go through the menopause, lose more bone in their lifetime than men; 50 per cent in females vs 35-40 per cent in males.
The fracture risk assessment tool ‘FRAX’ developed in 2008 provides a prediction tool for assessing an individual’s risk of fracture in order to provide general clinical
27 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
TABLE 2: Modi able and non-modi able risk factors for osteoporosis.
Source: Mayo Clinic and NIH
guidance for treatment decisions. It has been adapted to the Irish population using estimates about life expectancy and fracture incidence in Ireland. The Irish FRAX tool has been calibrated using national hip fracture incidence data and Irish mortality rates. FRAX computes the 10-year probability of a hip fracture or a major osteoporotic fracture. Eight identifiable risk factors shown to improve the prediction of fracture risk are included in FRAX including age, family history of hip fracture, glucocorticoid use, current smoking, alcohol use, and rheumatoid arthritis. Individually, the presence of these risk factors are shown to increase the risk of hip fracture at least 1.5 to two-fold after adjustment for BMD. FRAX has some limitations, as it only accepts one risk factor; while many cases of osteopaenia and osteoporosis have multiple risk factors, which can significantly increase the risk of fractures.
A DXA scan is used to measure BMD of the spine and hips which helps to assess the risk of bone fractures. DXA scans are most commonly used for diagnosing osteoporosis and assessing the risk of osteoporosis developing. BMD is mainly described as T-score, which represents the standard deviation (SD) number by which the BMD in an individual di ers from the mean value expected in young healthy individuals. T-scores should only be used in the diagnosis of adults over 21 years of age.
According to the World Health Organisation (WHO) criteria, osteoporosis is defined as a BMD that lies 2.5 SD or more below the average value for young healthy women (a T-score of < − 2.5 SD). The WHO classifies T-scores as follows: Above -1 SD is normal. Between -1 and -2.5 SD is classed as osteopaenia. Below -2.5 SD is classed as osteoporosis.
DXA also provides the patient’s Z-score, which reflects a value compared with that of persons matched for age and sex. Z-scores should be used in premenopausal women, men younger than 50 years, and children. Z-score values of -2.0 SD or lower are defined as below the expected range for age and those
above -2.0 SD as within the expected range for age.
Osteopaenia is the early stage of osteoporosis and places a person at risk of developing osteoporosis. The Irish Osteoporosis Society divides osteopaenia into three categories:
1. Mild osteopaenia is a T-score of -1 to -1.49 and usually requires lifestyle changes; however, causes should be investigated and addressed.
2. Moderate osteopaenia is a T-score of -1.5 to -1.9 which usually requires lifestyle changes. Causes should be found and addressed and the person may require medication, depending on the cause, or if they have had a fragility fracture.
3. Marked osteopaenia is a T-score of -2 to -2.49, which requires lifestyle changes. Causes should be found and addressed and the person may require medication, depending on the cause, or if they have had a fragility fracture.
causes of osteoporosis, age, DXA scan results and medical history. Assessment of bone markers before and at three and six months after the commencement of treatment will give an earlier indication of the response to treatment.
All postmenopausal women and individuals at risk of osteoporosis should be encouraged to maintain a healthy weight, ensure adequate calcium, vitamin D and protein intake and avoid excessive alcohol consumption and smoking. They should participate in appropriate exercise to improve muscle strength, balance, and maintain bone mass and utilise measures that prevent falls.
Calcium and vitamin D supplements
Treatment
Osteoporosis is treatable and fractures are preventable. The primary goal of osteoporosis therapy is to reduce the risk of fracture. A comprehensive osteoporosis treatment programme includes a focus on proper nutrition, exercise, and safety issues to prevent falls that may result in fractures. In addition, medication to slow or stop bone loss, increase bone density, and reduce fracture risk may be prescribed. The treatment selected for each individual is based on their risk of fracture or re-fracture,
Calcium and vitamin D are essential for the prevention and treatment of osteoporosis. Bone is a major store of calcium and phosphate. Every cell in the body requires calcium. Vitamin D helps to regulate cell growth and the immune system and is essential for the absorption of calcium. It increases the body’s ability to absorb calcium by 30-80 per cent. Vitamin D is the only vitamin required by the body that does not have to be consumed through food or supplements as it is manufactured through the skin, when exposed to sunlight. Supplements are generally only recommend when the daily amounts of calcium and vitamin D from dietary sources are not being met. Many people, however, do not get the recommended amounts of vitamin D through food or sunlight, and supplements are often recommended. In Ireland the RDA of calcium is 800mg/day for children, adults and older people, increasing to 1200mg for teenagers, pregnant and lactating women. The National Osteoporosis Foundation in the US recommends 1,000mg-1200mg calcium and 800-1,000IU of vitamin D for adults aged 50 years and older.
The richest sources of calcium in the diet are milk, cheese and yogurt. Three servings a day help meet the calcium needs of an adult or child and five servings are recommended during adolescence and pregnancy. Smaller amounts of calcium may be obtained from green vegetables, bread and sardines. The
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 28
A comprehensive osteoporosis treatment programme includes a focus on proper nutrition, exercise, and safety issues to prevent falls that may result in fractures

bioavailability of calcium from non-dairy sources is lower. Foods fortified with calcium and vitamin D can also be useful. A limited number of foods such as oily fish, egg yolks, liver, and fortified dairy products also provide vitamin D.
Due to Ireland’s northerly latitude and the use of sunscreens, vitamin D production from UVB light is compromised. Together with poor dietary intake, this has contributed to widespread sub-optimal vitamin D status. Low vitamin D status (25-hydroxyvitamin D <50nmol/l) is prevalent in Ireland, particularly in postmenopausal women. Dietary sources should be encouraged in all ages. Calcitriol is a vitamin D analogue. It is licensed for the treatment of established post-menopausal osteoporosis. Patients should have serum calcium and creatinine monitored for hypercalcaemia. Malnourished adults particularly those with an inflammatory condition are at increased risk of bone loss. Particular attention should be paid to the calcium intakes of individuals at risk of osteoporosis due to conditions such as coeliac disease, malabsorption or inflammatory bowel disease.
Hormone replacement therapy (HRT)
Oestrogen replacement for women going through the menopause can help to maintain bone density and reduce fracture rates while they are on the treatment. There is a direct relationship between the lack of oestrogen after menopause and the development of osteoporosis. Early menopause before age 45 and any long phases in which the woman has low hormone levels and no or infrequent menstrual periods can cause loss of bone mass. Oestrogen therapy and oestrogen with progesterone hormone therapy are approved for the prevention of osteoporosis in postmenopausal women provided there are no contraindications. HRT may not be suitable for people who have a history of breast cancer in their family, particularly in early menopausal patients or patients who have had a history of deep vein thrombosis.
Selective oestrogen receptor modulators (SERMS)
SERMs (raloxifene) work in a similar manner
to oestrogen on bone, by preventing bone loss in postmenopausal women who do not have hot flushes and provided there are no other contraindications. It is used for the prevention and treatment of osteoporosis in postmenopausal women and to reduce risk of invasive breast cancer in postmenopausal women at high risk or with osteoporosis. Raloxifine helps to maintain bone density and reduce fracture rates, specifically at the spine. It is administered as a 60mg tablet once daily. It can be taken with or without food or drink and at the same time as calcium and vitamin D supplements. Appropriate weightbearing exercise is also necessary.
Monoclonal antibodies
Denosumab is a monoclonal antibody, which binds to RANK ligand, inhibiting the maturation of osteoclasts, therefore protecting the bone from degradation. It is indicated for the treatment of osteoporosis in post-menopausal women and in men at increased risk of fractures. In postmenopausal women denosumab reduces the risk of vertebral, non-vertebral and hip fractures. it is also indicated for the treatment of bone loss associated with hormone ablation in men with prostate cancer at increased risk of fractures. It is the first choice of drug for those at high risk of hip fracture or who have had a hip fracture over the age of 75 with T-scores < −2.5 at femoral neck or with a humeral fracture. The recommended dose is 60mg administered as a single subcutaneous injection once every six months into the thigh, abdomen or upper arm. Patients must be adequately supplemented with calcium and vitamin D.
Bisphosphonates
Bisphosphonates also known as antiresorptive medications are nonhormonal drugs which help maintain bone density and prevent further bone loss. These medications are potent inhibitors of bone resorption and mainly increase the BMD of trabecular bones. Bisphosphonates are poorly absorbed from the GI tract. They should be taken alone on an empty stomach first thing in the morning with at least 250mls of water. After administration, the patient
should not have food, drink, medications, or supplements for at least a half-hour.
PTH
Parathyroid hormone (PTH) teriparatide is a recombinant human parathyroid hormone and a bone-forming agent that stimulates the formation of new bone. It is a ‘high tech’ medication that can only be prescribed by a consultant. It is given as a daily-subcutaneous injection in the thigh or abdomen for 24 months. The patient should then have a repeat DXA scan and a new treatment plan should be implemented at the end of the course of treatment. PTH is usually recommended for those with severe osteoporosis or fractures and those who cannot tolerate other medications. It can help with the pain of vertebral fractures and the reduction of vertebral and non-vertebral fractures in women.
Other treatments
Other treatments for osteoporosis can include kyphoplasty, a surgical treatment involving a balloon being placed into the fractured vertebrae, followed by ‘bone cement’ being injected into the balloon, or vertebroplasty, a non-surgical treatment involving a needle with ‘bone cement’ inserted into the fractured body of the vertebrae under imaging guidance. The decision to perform these techniques is made by a multidisciplinary team to ensure that this is the correct approach to managing the bone collapse.
Pain treatment
Many of the consequences of osteoporosis, particularly vertebral fractures, are associated with severe pain. Patients with established osteoporosis should be treated for pain relief and physiotherapy o ered for the secondary e ects of osteoporosis. Pharmaceutical and non- pharmaceutical measures can be used to alleviate pain. Patients should be advised of all the options, and encouraged to try di erent approaches until they find what works best for them. ■
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 30
available on request
See www.irishosteoporosis.ie for more information References
The home of Irish CPD Free, independent CPD for doctors, nurses and pharmacists…all under one roof medilearning A B C A B C pharmacistcpd doctorcpd nursecpd A B C
Diabetes and the kidney in 2020; glimmers of hope in the face of a global pandemic
AUTHORS: Dr Ross Doyle, Special Lecturer in Medicine, Clinical Research Fellow, UCD School of Medicine, and Prof Catherine Godson, Full Professor of Molecular Medicine, and Director of the Diabetes Complications Research Centre, Conway Institute and UCD School of Medicine
Often when we learn about a new disease when practising medicine, it is a rare, niche disorder, which we are prompted to read up about because we have come across a patient with it. Inevitably, the two sentences remembered from Wikipedia are quickly overshadowed by the knowledge brought to the table by the patient themselves. 2020, however, has provided us all with an opportunity to learn about a new disease and the resources available to us have been diverse; observations of a patient’s breathing from the end of the bed (through a visor), rapidly produced ‘scientific’ publications sometimes with dubious methodology, frequent briefings from public health physicians, and unfortunately, mixed messaging from self-appointed experts in the wider media. Diabetes and kidney disease have emerged as important comorbidities, which seem to impact outcomes in patients with Covid-19. Are we recognising this as an epi-phenomenon of both diabetes and kidney disease being common, chronic diseases, particularly among the groups termed ‘vulnerable people’, or are there features specific to these diseases that impact the natural course of the Covid-19 illness? Nearly half a billion people live with diabetes worldwide and between 11-15 per cent of adults have chronic kidney disease (CKD). Undoubtedly therefore, we were going to see plenty of people in these groups get Covid-19. Some evidence does point towards these populations developing more severe illness and there are some interesting suggestions as to why this may be.
Diabetes and kidney disease and the risk of Covid-19 infection
Reports in the early months of the
pandemic were conflicting on whether there was an increased incidence of Covid-19 in persons with diabetes. With di ering testing approaches, and variable sampling in di erent studies, it is clear how incidence rates in persons with diabetes may vary widely from study to study. However, a consistent finding has been that diabetes is overrepresented in people who get severely ill, require ITU treatment, and ultimately die. In a retrospective study of
over 7,000 patients in China with Covid-19, the mortality rate was approximately 7.8 per cent in people with diabetes, compared with 2.7 per cent in those without. These authors also found a significant di erence in survival in patients with ‘well-controlled’ diabetes, compared to ‘poorly-controlled’. The so-called well-controlled group had a median in-hospital blood glucose level of 6.4mmol/L (IQR 5.2-7.5), compared with a median in-hospital blood glucose level of 10.9 (IQR 7.6-14.3); median HbA1c was 7.3 per cent in the well-controlled group and 8.1 per cent in the poorly-controlled group.
Acute kidney injury (AKI) has been reported with variable rates in di erent studies, but of 5,449 patients followed in 23 hospitals in New York, US, 36.6 per
cent developed AKI. This is higher than the rates of AKI we have traditionally considered to occur in hospitalised patients, based on historical data, which is closer to 20 per cent. With Covid-19, there appears to be a strong relationship between the development of respiratory failure, particularly the need for mechanical ventilation, and the development of AKI. The timing of AKI diagnosis also appeared to align with the time of intubation. Approximately one-in-20 of all patients hospitalised with Covid-19 developed AKI so severe as to require renal replacement therapy (RRT). More than one-in-five patients who were mechanically ventilated required dialysis support. The high rate of AKI and requirement for dialysis circuitry led to international shortages of supplies for acute dialysis, which led to national and international organisations devising contingency plans for alternative modes of RRT delivery in the ITU. These shortages and concerns did not receive the same media attention as the shortages of mechanical ventilators, which was widely reported in the media.
A post-mortem series of 63 patients dying with Covid-19 identified SARS-CoV-2 RNA in 38 patients, which was associated with a reduced survival time, suggesting that there may indeed be a role played by direct viral infection and replication within kidney cells.
The presence of underlying CKD appears to be associated with more severe Covid-19 disease, as suggested by a metaanalysis that included 1,389 patients. Further granularity has been provided
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 32
Diabetes and kidney disease have emerged as important comorbidities, which seem to impact outcomes in patients with Covid-19
by an analysis of over 10,000 deaths from Covid-19, highlighting a graded association between severity of underlying kidney dysfunction and risk of Covid-19-related death. The hazard ratio (HR) for death in patients with eGFR <30ml/min/1.73m2 was almost double that of the HR for death in people with underlying respiratory disease, chronic heart disease or obesity.
into respiratory cells, and thereby act to increase susceptibility to infection. ACE inhibitors do not inhibit ACE2 because ACE and ACE2 are structurally diverse with di erent active sites. Nevertheless, treatment of patients with type 1 and type 2 diabetes with either ACE inhibitors or ARBs has previously been shown to increase ACE2 expression in renal and cardiovascular systems.
The Lancet reported a case of the administration of recombinant soluble ACE2 to a patient with type 2 diabetes and Covid-19, administered daily for seven days, commencing on the ninth day after symptom onset. Circulating levels of angiotensin II decreased following treatment with recombinant ACE2.
A number of potential explanations as to why patients with diabetes and kidney disease were doing so badly have been proposed. These include factors related to how the virus itself enters cells, the lowgrade chronic inflammation and altered cytokine production in these diseases, and abnormalities of the immune systemendothelium interface.
Angiotensin converting enzyme 2
A slew of publications in March this year linked Covid-19 to angiotensin converting enzyme 2 (ACE2), an enzyme which is responsible for the degradation of angiotensin II. It became apparent that this protein acts as a receptor for the spike protein of the coronavirus, and could mediate its entry to cells.
Host cell TMPRSS2, a serine protease is responsible for ‘priming’ the viral spike protein for interacting with ACE2 and cellular entry. ACE2 is widely expressed, on endothelium, including in the lung and kidney. Variability of ACE2 expression, particularly in response to treatment with angiotensin II type 1 receptors blockers (ARBs), which can increase ACE2 expression, led to the hypothesis that these medications could increase the incorporation of the virus
Initially, discussions on online fora suggested that these drugs be discontinued, so as to avoid increased expression of the protein responsible for viral entry. It quickly became apparent that such recommendations would be unsubstantiated and statements from international societies soon followed, emphasising that there was no clinically relevant evidence available to us to recommend widespread changes to the prescription of these drugs. Early results of a small open-label randomised trial conducted in Brazil, and presented at the annual European Society of Cardiology (ESC) Congress in early September, involving 649 patients did not identify any reduction in mortality in participants randomised to discontinuation of ACEi/ ARB therapy upon hospital admission with Covid-19 (NCT04364893).
The ACE2 protein has essential roles in regulation of the renin angiotensin aldosterone system. There is evidence that infection of the cell with virus results in downregulation of the ACE2 leading to reduced activity of ACE2 in the infected organs. ACE2 generates a peptide Ang 1-7, which typically balances vasoconstrictive responses to Ang 2, therefore decreased ACE2 activity in response to SARS-CoV-2 binding has been postulated to contribute to the pathology of Covid-19. There is also evidence from mice that depletion of ACE2 worsened acute lung inflammation in other models of disease, including sepsis, acid aspiration and endotoxinaemia. Treatment of mice with losartan, an ARB, which we know can increase ACE2 expression, resulted in alleviation of the lung inflammation.
Inflammatory cytokines and viral load decreased. The patient was extubated after 21 days, and discharged from hospital after 57 days. We can only speculate as to the role played by this intervention in dictating the course of the patient’s illness.
Angiotensin plays an important role in tissue inflammation. We do not understand fully the e ects of the host cell downregulating ACE2 expression following viral infection. Is it the case that this is occurring to prevent further viral infection, but at the expense of loss of regulation of angiotensin-mediated inflammatory e ects? A large cohort study of 19,486 patients with Covid-19 did not identify any altered risk of needing ITU care in persons previously prescribed ACE inhibitors or ARBs. Subtle di erences in di erent ethnic groups in this study were evident, however. This underscores the complexity of the host inflammatory response to viral infection, that we do not fully understand all of its nuances, but also that large-scale studies with heterogenous populations may dilute down potentially meaningful clinical results.
Interestingly, dipeptidyl peptidase-4, which is a drug target of the gliptin class of diabetes medications, is a receptor for MERS-CoV interacting with its spike glycoprotein. Its ability to interact with SARS-CoV-2, or whether any of the small molecule inhibitors prescribed for the management of diabetes can modify viral binding remain unknown.
Abnormalities in cytokine production
A state of chronic low-grade inflammation and abnormalities of cytokine profiles is recognised in people with diabetes and
33 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
Approximately one-in-20 of all patients hospitalised with Covid-19 developed AKI so severe as to require renal replacement therapy (RRT)
chronic kidney disease. We recognise that many pro-inflammatory markers are elevated in these states, including interferon-gamma, IL-1 family, IL-6, IL-8, TNF-alpha and others. We also know that people with diabetes have impaired adaptive immune response, with delayed Th1 cell-mediated immunity and a late hyperinflammatory response. What happens when this inflammatory milieu is challenged with SARS-CoV-2 infection? There is some evidence that the chronic inflammation seen in diabetes is amplified further upon SARS-CoV-2 infection. Investigations in diabetic mice infected with MERS-CoV highlighted alterations in CD4+ T-cells and cytokine responses, findings which have now been replicated in patients with diabetes and Covid-19. The low lymphocyte count characteristic of the viral infection is also seen in people with diabetes, but absolute neutrophil counts were higher when compared to patients without diabetes.
A ‘cytokine storm’ is described as part of the Covid-19 illness, and has been well described in a number of other scenarios since the 1990s. A cascade of auto-amplifying cytokine production because of a dysregulated host response to a trigger is believed to occur, resulting in accelerated injury in multiple organs, worsening lymphopenia and elevated inflammatory markers. Elevations in IL-1, IL-2, IL-6, TNF-alpha and interferongamma are described as part of this ‘storm’. The manifestations described a ect almost all organ systems, and include elevations in organ-specific biomarkers, including liver enzymes, troponins, creatinine as well as manifestations like palpitations, myocardial infarctions, AKI, strokes, diarrhoea, and worsening respiratory failure, among others. Some authors suggest that the cytokine storm, not seen in all patients with Covid-19, is more likely to develop in people with diabetes because of the already dysregulated inflammatory response and low-grade inflammation characteristic of the disease. This may go some way to explaining the increased severity of illness
and higher mortality experienced by people with diabetes and Covid-19.
The RECOVERY trial, identifying the role of corticosteroids in a select subset of patients with Covid-19, brings with it challenges in terms of glycaemic control, particularly in patients with diabetes. Mortality was decreased in patients who were receiving invasive mechanical ventilation or oxygen, but not among those receiving no respiratory support. A quarter of the participants had diabetes. Diabetes UK and the National Inpatient Diabetes Covid-19 Response Group in response to the results of this trial have put together helpful advice sheets for maintaining glycaemic control and insulin adjustments for patients with and without diabetes receiving this treatment.
Endothelium interface
It may not be considered surprising that patients with diabetes and kidney disease experienced increased severity of illness and mortality with Covid-19. We have long appreciated the complex and systemic nature of these diseases, and their vulnerability to infection, multi-organ dysfunction and critical illness. However, we may not have predicted the importance of endothelial dysfunction and the abundance of clinical manifestations of this, seen as part of this pandemic. Endothelial dysfunction is a well-recognised phenomenon in diabetes and in kidney disease. The large surface area of the endothelium in the kidney, with its two sets of capillary beds in series, may mean the kidney as an organ is more susceptible to a pathological process involving endothelial cells. ACE2 is widely expressed in endothelial cells, but also in kidney proximal tubular cells and glomerular epithelial cells.
Interestingly, patterns of ACE2 expression in kidney tissue in animal models of injury vary. Studies in diabetic mice models suggest increased ACE2 expression, while other kidney injury models, such as models of hypertension, ischaemiareperfusion or subtotal nephrectomy,
show decreased ACE2 expression. This highlights further the complexities of the renin angiotensin aldosterone system, and reminds us that the more we learn about it the more we recognise how much there is we don’t know and the importance of access to human tissue for research.
Endothelial dysfunction and endothelialitis can be induced in many organs as part of Covid-19. Venous and arterial thrombi have been identified as major contributors to mortality in patients with Covid-19. Evidence of vessel occlusion in the capillary network of the hands and feet, with chilblain-like lesions, termed ‘Covid toes’ allows us to see clearly the manifestations of such endothelial dysfunction. This can help us think conceptually about what might be happening in the sluggish vascular network of the vasa recta of the kidney. Some authors suggest that the endothelial cell and immune cell interaction in Covid-19 could represent an important link to explain the added morbidity and adverse outcomes seen in patients with diabetes and kidney disease.
SGLT2 inhibitors in patients without diabetes
Setting aside the complexities and added morbidity experienced by patients with diabetes and kidney disease in the context of Covid-19, these populations have some hope that we will see a paradigm shift in approaches to their management in the future. In August of this year we learned of the results of a trial studying SGLT2 inhibitors in persons with kidney disease, irrespective of diabetes status. These drugs have been finding their feet in the diabetes world in recent years. Their mechanism of action involves the prevention of uptake of glucose from the glomerular ultrafiltrate in the proximal tubule of the kidney.
However, it has been clear from the major trials of these drugs in diabetes that other forces must be at play, because the improvements and benefits seen outweigh what would be expected
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 34
given their modest glucose-lowering effects. Importantly, these agents act independent of insulin secretion.
Randomised controlled trials of these agents have confirmed a reduced risk of major adverse cardiac events, particularly in patients with a history of or at high risk for cardiovascular events.
The EMPA-REG OUTCOME study evaluated 7,020 participants who had type 2 diabetes and a history of cardiovascular disease. The CANVAS study included 10,142 patients with type 2 diabetes considered at high risk for cardiovascular events. In both of these studies, the rate of major adverse cardiac events (MACE) was lower than placebo. The DECLARE-TIMI 58 study included 17,160 patients with type 2 diabetes, the majority of whom did not have existing atherosclerotic cardiovascular disease. In this trial, the SGLT2 inhibitor (dapagliflozin) was noninferior to placebo.
In addition to cardiovascular benefits, clear benefits in terms of progression of kidney disease have been demonstrated. A meta-analysis, which included 40 trials and 29,954 patients, confirmed that SGLT2 inhibitors in patients with type 2 diabetes and kidney disease were associated with preservation of renal function and prevented death from kidney disease. People with type 2 diabetes and kidney disease treated with these agents are also less likely to progress towards needing dialysis. Strikingly, whereas the cardiovascular benefits of these agents in people with diabetes seem limited to those at high risk of, or with, pre-existing atherosclerotic cardiovascular disease, a meta-analysis looking at risk of progression of renal disease has confirmed that there is a 45 per cent reduction in the risk of progression of renal disease, a benefit that is similar in patients with and without atherosclerotic cardiovascular disease.
This paved the way for the DAPA-CKD trial to test the hypothesis that the
SGLT2 inhibitor (dapagliflozin) was superior to placebo at reducing renal and cardiovascular events in patients with CKD (irrespective of diabetes status), who were already on established therapy with either an ACE inhibitor or ARB. The primary composite outcome here was worsening of kidney function (a sustained drop in eGFR by more than half, or developing end stage kidney disease), or death due to kidney or cardiovascular disease. A total 4,304 patients were studied, who had evidence of CKD (with eGFR ranging from 25-75
Bariatric surgery and kidney disease
Bariatric surgery has emerged as an important intervention for patients with type 2 diabetes and obesity. We are now coming to appreciate that the benefits of this intervention extend beyond improvement in glycaemic control and amelioration of underlying diabetes. A large observational cohort study in Sweden that followed over 5,000 individuals with type 2 diabetes undergoing gastric bypass has highlighted improvements in kidney function. Harnessing the large registry data available in Sweden the researchers were able to match these patients to approximately 5,000 patients who did not undergo surgery and identify that patients who underwent surgery were much less likely to develop albuminuria, or halving of their eGFR. Some of the proposed mechanisms underpinning this seen benefit include decreased levels of glucagon-like peptide 1 (GLP1), decreases in insulin release driven by incretin hormones following a meal, improvements in blood pressure control, decreased levels of renin, angiotensin II, endothelin and neprilysin.
Conclusion
ml/min/1.73m 2 and albuminuria). After a median follow-up time of 2.4 years, the HR for the primary endpoint was 0.61 (0.51-0.72), showing that dapagliflozin could reduce the risk of worsening kidney function, or death from cardiovascular or kidney disease in patients with CKD. The patients in this study were already taking the maximum tolerated doses of ACE inhibitors or ARBs. The benefits were consistent in those participants who had type 2 diabetes and those who did not.
These important results represent a turning point for therapies in CKD, which have remained largely stagnant since the 1990s in the face of rising prevalence of the disease.
Times are certainly changing for patients living with CKD and diabetes. Amid the tragedy witnessed throughout 2020 and the excess morbidity and mortality these populations have been burdened with in the context of Covid-19, there is some hope that we are seeing a change in the tide of their treatment approaches. If anything, this should give us hope as we look forward to a world where we move towards living without Covid-19 and bring the chronic disease management of these patients back into focus. We will hopefully see more patients prescribed SGLT2 inhibitors who do not also need a glucometer, and think about resourcing obesity surgery in order to avoid the huge costs of longterm dialysis therapy. ■
References on request
35 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
A meta-analysis, which included 40 trials and 29,954 patients, confirmed that SGLT2 inhibitors in patients with type 2 diabetes and kidney disease were associated with preservation of renal function and prevented death from kidney disease
Treating type 2 diabetes and obesity: How to treat a twin pandemic
AUTHORS: Dr Karl Ne ; Consultant Endocrinologist and Obesity Physician, and Therese Coleman; Specialist Obesity Dietician, Obesity Complications Clinic, St Vincent’s Private Hospital, Dublin
Type 2 diabetes and obesity commonly coexist. In fact, these two conditions can be considered as a twin pandemic a ecting increasing numbers of people in Ireland. Many of the treatments for type 2 diabetes can inadvertently exacerbate concurrent obesity. In theory, the exacerbation of obesity can result in worsening insulin resistance which would make managing type 2 diabetes more di cult, potentially creating a vicious cycle of weight gain and suboptimal glycaemic control.
Therefore, when treating type 2 diabetes, it is important to consider the e ect of this treatment on concurrent obesity. It is also important to select therapeutic interventions that can o er synergistic e ects on both glycaemic control and body weight. Insulin, sulphonylureas and pioglitazone can all exacerbate obesity when used to improve glycaemic control in type 2 diabetes. Other agents such as DPP4 inhibitors and metformin are weight neutral and can be safely used in people with both type 2 diabetes and obesity. However, some pharmacological agents used to treat type 2 diabetes can also be used to help treat obesity. Therefore, these agents should be considered second line after metformin in people with obesity-associated type 2 diabetes.
In addition to pharmacotherapy, specialist dietary interventions can be used to treat obesity and type 2 diabetes. Results from studies such as the DIRECT trial have shown that low calorie diets can improve glycaemic control in those with type 2 diabetes. In those with recent onset of type 2 diabetes, these interventions can sometimes induce remission of diabetes.
As well as the glycaemic benefits, these interventions can also e ectively treat obesity, and therefore should be considered in people who have concurrent obesity and
type 2 diabetes. However, these interventions should only be o ered in the context of specialist multidisciplinary care with medical support. At present, the multidisciplinary framework needed for implementation of such a therapeutic intervention remains in development in Ireland and therefore outside of specialist hospital-based clinics, these interventions are not widely available.
At present, available GLP-1 receptor agonists include semaglutide, liraglutide, and exenatide. These agents are given as once weekly, once daily, and twice-daily injections respectively. These are all administered as injectable therapy. Oral GLP-1 receptor agonists are in development. However, at the time of writing these are not commercially available.
In this short article, we will outline the three major interventions which can be used to treat both type 2 diabetes and obesity. Two of these, SGLT2 inhibitors and GLP-1 receptor agonists, can be prescribed safely in primary care. The third, specialist dietary interventions, should only be o ered within a specialist multidisciplinary pathway.
GLP-1 receptor agonists
GLP-1 receptor agonists improve glycaemic control via several mechanisms including augmentation of insulin secretion and modulation of glucagon secretion. They also have significant e ects on energy homeostasis by enhancing appetite control. Many people using GLP-1 receptor agonists will notice a reduction in hunger and food intake. Given these e ects, there is significant weight loss associated with GLP-1 receptor agonist therapy. The reduction in body weight varies significantly in users. An average weight reduction of 3kg is expected, but some users will lose significantly more and others may lose less.
Given that GLP-1 receptor agonists are given via injection, there is often a concern among patients that they may result in hypoglycaemia, as many people mistake them for an insulin therapy. However, given the glucose-dependent nature of GLP-1 receptor agonists glycaemic e ect, hypoglycaemia does not occur in people using GLP-1 receptor agonists in the same way as other agents, such as sulphonylureas, and so people using these agents do not need to inform their driving licensing authority that they are using them.
GLP-1 receptor agonists are e ective in improving glycaemic control in people with type 2 diabetes, and can reduce HbA1c by more than 1 per cent (11mmol/mol) when compared to placebo. In trials evaluating them alongside commonly used active comparators such as sulphonylureas, GLP-1 receptor agonists are at least as e ective in terms of HbA1c improvement.
While GLP-1 receptor agonists were originally designed and licensed to treat type 2 diabetes, the weight loss e ect noted above led to a range of studies investigating the use of GLP-1 receptor agonists as a primary treatment for obesity. There is now an abundance of randomised controlled data proving that these agents are e ective in the treatment of obesity. These obesity studies were completed in people with and without diabetes, and so GLP-1 receptor agonists can be safely used to treat obesity in people who do not have diabetes.
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 36
It is also important to select therapeutic interventions that can o er synergistic e ects on both glycaemic control and body weight
Most people who use these agents to treat obesity will achieve greater than 10 per cent weight loss at 12 months. However, there is a variance in e ect, and some people will have little or no weight loss. The weight loss e ect will be obvious within four months of treatment (presuming that dose titration has occurred and that the patient has received at least three months of full dose therapy). Therefore, if people have not achieved greater than 5 per cent weight loss after four consecutive months of treatment, then the therapy is unlikely to be e ective and should be discontinued.
The doses of GLP-1 receptor agonists used to treat type 2 diabetes are covered by the GMS, Long-term Illness Scheme, and DPS in Ireland. Higher doses of Liraglutide (3mg daily) are licensed for use in the treatment of obesity in people with or without concurrent diabetes. However, at the time of writing, this dose is not covered by any of the drug payment schemes.
Liraglutide 3mg daily is associated with greater weight loss than lower doses. Patients taking the highest doses of liraglutide lose significantly more weight than those taking lower doses, or other anti-obesity pharmacotherapy. An average of 8kg weight loss is expected with the 3mg dose, but there is a variance of e ect. Some people will lose less than this, and some people will lose significantly more. While it is impossible to predict the treatment response prior to initiation of therapy, the e ectiveness of the therapy will become obvious within four months of treatment. At that stage, if the patient has not lost more than 5 per cent of their bodyweight, then they are unlikely to have a significant weight loss response and the treatment should be discontinued. However, if they have achieved greater than 5 per cent bodyweight then it should be continued for as long as is tolerated.
Sodium glucose code transporter two inhibitors (SGLT 2 inhibitors)
SGLT2 inhibitors are currently available for the treatment of type 2 diabetes either as monotherapy or in combination with other established pharmacotherapy. This class of
agents act via the proximal tubule where mediation of glucose filtration occurs. SGLT2 inhibitors prevent resorption of glucose in the nephron and facilitates glycosuria. Given that SGLT2 inhibitors can only lower plasma glucose by inhibiting reabsorption of renally filtrated glucose, they are not expected to produce hypoglycaemia.
Glycaemic control can be improved with SGLT2 inhibitors, but as with GLP-1 receptor agonists, there is a variance of e ect. Reductions in HbA1c concentrations range from between 0.5 to 1 per cent (6mmol/ mol to 11mmol/mol). When assessed with active comparators, there is no clinically significant di erence between SGLT2
to be limited to those with established cardiovascular disease).
There are also well described renal benefits associated with SGLT2 inhibitor use. In patients with established diabetic kidney disease, as defined by albuminuria with or without reduced estimated glomerular filtration rate (GFR), the use of SGLT2 inhibitors reduces the rate of progression of chronic kidney disease and prevents the onset of end-stage kidney disease, therefore reducing the need for dialysis and/or renal transplantation. This is particularly obvious in those with estimated GFRs of 30 - 60mL/ min. Therefore, SGLT2 inhibitors should be considered as second-line agents in people with type 2 diabetes with established diabetic kidney disease, including those with a GFR of greater than 60mL/minute with microalbuminuria.
inhibitors and commonly used therapies such as sulphonylureas or DPP4 inhibitors. However, there are some additional benefits associated with SGLT2 inhibitors, which would promote their use as a second-line agent in certain populations.
Recent data has illustrated benefits in patients with heart failure. In cardiovascular disease outcome trials, SGLT2 inhibitors reduce the risk of major adverse cardiovascular events and hospitalisation for heart failure as compared with placebo. There is also a benefit in terms of risk reduction of myocardial infarction, cardiovascular death and stroke (although based on current evidence this e ect appears
In patients with type 2 diabetes and obesity, there is also a benefit with respect to weight management. SGLT2 inhibitors are associated with modest weight loss. A mean weight loss of 3kg is reported in randomised controlled data when compared to placebo. This can be more obvious in clinical practice when SGLT2 inhibitors are compared to the use of sulphonylureas or insulin, both of which are associated with weight gain. Therefore, in patients presenting with type 2 diabetes and obesity, SGLT2 inhibitors should be considered second or third line after metformin with GLP-1 receptor agonists.
Specialist dietary interventions
Specialist dietary interventions can be used to treat both type 2 diabetes and obesity, either concurrently or individually. Diets with 800kcal per day or less are consistent with the term very low-calorie diet. Diets with reduced calorie intakes, but with absolute intakes of over 800kcal per day, can be termed low-calorie diets.
The Diabetes Remission Clinical Trial (DIRECT) was completed in a primary care setting in the UK. This landmark study demonstrated that low-calorie dietary intervention, delivered in a primary care
37 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
This landmark study demonstrated that lowcalorie dietary intervention, delivered in a primary care setting with specialist support from dieticians and GPs, could deliver weight loss of 15kg or more in almost 25 per cent of participants
setting with specialist support from dieticians and GPs, could deliver weight loss of 15kg or more in almost 25 per cent of participants. Full remission of diabetes could be achieved in almost half of participants.
Very low-calorie diets and low-calorie diets are usually delivered as specially formulated food products, usually in the form of milkshakes, soups or nutritional bars. There are several studies showing that in people living with obesity, the use of low and very low-calorie diets can result in significant weight loss in the short to medium-term. In most studies, there is a significant drop-out rate as people find the restriction in energy intake di cult to tolerate, and can find associated side-e ects, such as constipation, to be intolerable.
However, in those who do tolerate the intervention, obesity can be successfully treated with a weight reduction of between 10-to-15 per cent body weight over 12 to 20 weeks of treatment. Weight maintenance following cessation of treatment can be challenging but is achievable. Weight regain appears to be mediated by physiological changes and metabolic adaptations that result in increased hunger and reduced basal metabolic rates following a period of reduced energy intake. However, despite these physiological and metabolic adaptations that promote weight regain, clinical studies have shown that weight loss maintenance is possible with ongoing dietary modification, the addition of pharmacotherapy, and concurrent exercise programmes. It should be noted that while exercise therapy may not be proven to significantly enhance weight loss during obesity treatment programmes, exercise therapy has been shown to assist with weight maintenance in the medium to long-term, which is why it is an integral element of obesity treatment programmes.
As well as being e ective obesity treatment in selected patients with obesity, low-calorie and very low-calorie diets can also be e ective in the treatment of type 2 diabetes. In the landmark Counterbalance study,
the investigators proved the concept that reducing fat mass in general would reduce fat infiltration of the pancreatic islet cells and result in an improvement in islet cell function. Counterbalance was completed in 30 subjects who had type 2 diabetes for more than eight years. In this group, 50 per cent achieved fasting blood glucose concentrations of less than 7mmol/l within eight weeks of use of a very low-calorie diet. Following the use of this very low-calorie diet, patient returned to an isocaloric diet, and maintained the improved glycaemic control for up to six months.
In DIRECT, the aim was to induce remission or improve control of type 2 diabetes using a low-calorie diet. In this study, the intervention was a low-calorie diet of between 800kcal and 900kcal per day which was prescribed for at least 12 weeks and continued for up to five months. Following this intervention, the participants progressed to a staged reintroduction of an isocaloric diet. A structured weight maintenance programme was then introduced and continued for up to 12 months. This intervention was compared with standard diabetes and obesity treatment, that was based on lifestyle advice and diabetic pharmacotherapy.
Almost 50 per cent of people in DIRECT achieved remission of their type 2 diabetes. This cohort had a diagnosis of type 2 diabetes within six years of recruitment to the study. Type 2 diabetes remission was defined as HbA1c of less than 6.5 per cent (48mmol per mole) when all diabetes pharmacotherapy had been discontinued for at least two months. Concurrent treatment of obesity was associated with greater glycaemic control, and those who lost most weight generally achieved greater glycaemic control and were more likely to achieve diabetes remission. In DIRECT, 24 per cent of people achieved more than 15kg of weight loss as compared to the control group. Overall weight reduction in the intervention group was 10kg.
While DIRECT demonstrated that such an intervention could be safely delivered
in the primary care setting, it is relatively resource intensive. Therefore, in the current Irish healthcare system this would not be an option for most people outside of hospital-based obesity clinics. However, given the reduction in requirements for pharmacotherapy, and the likely reduction in long-term microvascular disease as a result of improved glycaemic control, these interventions may prove to be cost neutral in the long-term and so the cost-benefit implications would result in wider application of specialist dietary interventions.
Conclusion
When treating patients who have concurrent type 2 diabetes and obesity, we need to be careful about how we proceed with treatment. Metformin should be the foundation of diabetic pharmacotherapy. However, in patients with both type 2 diabetes and obesity, we would suggest that GLP-1 receptor agonists and SGLT2 inhibitors would be considered as secondand third-line pharmacotherapy. As in all diabetes care, pharmacotherapy should be applied in the context of lifestyle modification including balanced healthy eating and regular exercise.
Specialist dietary interventions are proven to be e ective treatments in both type 2 diabetes and obesity, and recent studies have shown them to be safely deliverable in a primary care setting. While this is currently not available in Ireland, it should be a goal that we work towards, in the context of a wider obesity treatment strategy that works via integrated care networks across hospital and community clinics.
Many challenges face Irish healthcare, but few are as prevalent as the twin pandemic of type 2 diabetes and obesity. Prevention should remain the focus of our approach as a society and as a healthcare system, but when type 2 diabetes and obesity have developed the only approach that is appropriate is to treat with specialist disease specific interventions. ■
References on request
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 38
The journey to triple protection in T2D starts at the kidneys


Choose INVOKANA – the only SGLT2i licensed to offer your T2D patients triple protection against renal events, CV events and elevated HbA1c1–4 HbA1c


INVOKANA® (canagliflozin) 100 mg & 300 mg film-coated tablets. PRESCRIBING INFORMATION. REPUBLIC OF IRELAND. Please refer to the Summary of Product Characteristics (SmPC) before prescribing. INDICATIONS: The treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise as monotherapy when metformin is considered inappropriate due to intolerance or contraindications, or in addition to other medicinal products for the treatment of diabetes. DOSAGE & ADMINISTRATION:
Adults: recommended starting dose: 100 mg once daily. In patients tolerating this dose and with eGFR ≥ 60 mL/min/1.73 m2 needing tighter glycaemic control, dose can be increased to 300 mg once daily. For oral use, swallow whole. Caution increasing dose in patients ≥ 75 years old, with known cardiovascular disease or for whom initial canagliflozininduced diuresis is a risk. Correct volume depletion prior to initiation. When add-on, consider lower dose of insulin or insulin secretagogue to reduce risk of hypoglycaemia. Children: no data available. Elderly: consider renal function and risk of volume depletion. Renal impairment: for the treatment of diabetic kidney disease (DKD) as add on to standard of care (SOC) (e.g. ACE inhibitors or ARBs), initiate with 100 mg dose. The glycaemic lowering efficacy of canagliflozin is reduced in patients with moderate renal impairment and likely absent in severe renal impairment. If eGFR falls below 60 mL/min/1.73 m2 during treatment, adjust or maintain dose at 100 mg once daily.
Total daily dose of canagliflozin

Initiate with 100 mg
If tolerating 100 mg and needing additional glycaemic control, increase dose to 300 mg
Initiate with 100 mg
If already taking Invokana – continue 100 mg
Initiate with 100 mg
If already taking Invokana – continue 100 mg
Do not initiate
If already taking Invokana – continue 100 mgc
a Consider addition of other anti-hyperglycaemic agents if further glycaemic control is needed
b With urinary albumin/creatinine ratio > 300 mg/g c Continue until dialysis or renal transplantation Hepatic impairment: mild or moderate; no dose adjustment. Severe; not studied, not recommended. Hepatic impairment: mild or moderate; no dose adjustment. Severe; not studied, not recommended. CONTRAINDICATIONS: Hypersensitivity to active substance or any excipient. SPECIAL WARNINGS & PRECAUTIONS: Not for use in type 1 diabetes. Renal impairment:
regardless of pretreatment, patients on canagliflozin had an initial fall in eGFR that attenuated over time. eGFR < 60 mL/min/1.73 m2: higher incidence of adverse reactions associated with volume depletion particularly with 300 mg dose; more events of elevated potassium; greater increases in serum creatinine and blood urea nitrogen (BUN); limit dose to 100 mg once daily. Not studied in severe renal impairment. Monitor renal function prior to initiation and at least annually. Volume depletion: caution in patients for whom a canagliflozin- induced drop in blood pressure is a risk (e.g. known cardiovascular disease, eGFR < 60 mL/min/1.73 m2, anti-hypertensive therapy with history of hypotension, on diuretics or elderly). Not recommended with loop diuretics or in volume depleted patients. Monitor volume status and serum electrolytes. Diabetic ketoacidosis (DKA): rare DKA cases reported, including life-threatening and fatal. Presentation may be atypical (blood glucose <14mmol/L). Risk appears higher in patients with moderate to severe decrease in renal function who require insulin. Consider DKA in event of non-specific symptoms. If DKA is suspected or diagnosed, discontinue Invokana treatment immediately. Interrupt treatment in patients who are undergoing major surgical procedures or have acute serious medical illnesses. Monitoring of (preferably blood) ketone levels is recommended in these patients. Consider risk factors for development of DKA before initiating Invokana treatment. Elevated haematocrit: careful monitoring if already elevated. Genital mycotic infections: risk in male and female patients, particularly in those with a history of GMI. Lower limb amputation: Consider risk factors before initiating. Monitor patients with a higher risk of amputation events, counsel on routine preventative foot care and adequate hydration. Consider discontinuing Invokana when events preceding amputation occur (e.g. lowerextremity skin ulcer, infection, osteomyelitis or gangrene). Necrotising fasciitis of the perineum (Fournier’s gangrene): post-marketing cases reported with SGLT2 inhibitors. Rare but serious, patients should seek medical attention if experiencing symptoms including pain, tenderness, erythema, genital/perineal swelling, fever, malaise. If Fournier’s gangrene suspected, Invokana should be discontinued, and prompt treatment instituted. Urine laboratory assessment: glucose in urine due to mechanism of action. Lactose intolerance: do not use in patients with galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. Sodium: essentially “sodium-free”. INTERACTIONS: Diuretics: may increase risk of dehydration and hypotension. Insulin and insulin secretagogues: risk of hypoglycaemia; consider lower dose of insulin or insulin secretagogue. Effects of other medicines on Invokana: Enzyme inducers (e.g. St. John’s wort, rifampicin, barbiturates, phenytoin, carbamazepine, ritonavir, efavirenz) may decrease exposure of canagliflozin; monitor glycaemic control. Consider dose increase to 300 mg if administered with UGT enzyme inducer. Cholestyramine may reduce canagliflozin exposure; take canagliflozin at least 1 hour before or 4-6 hours after a bile acid sequestrant. Effects of Invokana on
LICENCE EXTENSION
INVOKANA now includes evidence for the treatment of diabetic kidney disease1




other medicines: Monitor patients on digoxin, other cardiac glycosides, dabigatran. Inhibition of Breast Cancer Resistance Protein cannot be excluded; possible increased exposure of drugs transported by BCRP (e.g. rosuvastatin and some anti-cancer agents). PREGNANCY: No human data. Not recommended. LACTATION: Unknown if excreted in human milk. Should not be used during breast-feeding. SIDE EFFECTS: Very common (≥1/10): vulvovaginal candidiasis, hypoglycaemia in combination with insulin or sulphonylurea. Common (≥1/100 to <1/10): balanitis or balanoposthitis, urinary tract infection (including pyelonephritis and urosepsis), constipation, thirst, nausea, polyuria or pollakiuria, dyslipidemia, haematocrit increased. Uncommon (<1/100) but potentially serious: necrotising fasciitis of the perineum (Fournier’s gangrene) (frequency not known), anaphylactic reaction, diabetic ketoacidosis, syncope, hypotension, orthostatic hypotension, urticaria, angioedema, bone fracture, renal failure (mainly in the context of volume depletion), lower limb amputations (mainly of the toe and midfoot.
Refer to SmPC for details and other side effects. LEGAL CATEGORY:





POM. PACK SIZES & MARKETING AUTHORISATION NUMBER(S): Invokana 100 mg film-coated tablets: 30 tablets; EU/1/13/884/002. Invokana 300 mg film-coated tablets: 30 tablets; EU/1/13/884/006.
MARKETING AUTHORISATION HOLDER: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. ® INVOKANA is a registered trade mark of Janssen-Cilag International NV and is used under licence. © 2020 Napp Pharmaceuticals Limited.










Adverse events should be reported to: HPRA Pharmacovigilance, Earlsfort Terrace, IRL – Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
Adverse events should also be reported to Mundipharma Pharmaceuticals Limited on drugsafetyJNJ@mundipharma-rd.eu or by phone on 01 2063800 (1800 991830 outside office hours)
FURTHER INFORMATION IS AVAILABLE FROM: Mundipharma Pharmaceuticals Limited, Millbank House, Arkle Road, Sandyford, Dublin 18. For medical information enquiries, please contact medicalinformation@ mundipharma.ie UK/INV-18203(3)
IRE/INVK-20206a





Date of Preparation July 2020

CV: cardiovascular; HbA1c: haemoglobin A1c; SGLT2i: sodium-glucose cotransporter 2 inhibitor; T2D: type 2 diabetes. ®MUNDIPHARMA and the ‘mundipharma’ logo are registered trademarks of Mundipharma. Invokana® is a registered trademark of Johnson & Johnson. IRE/INVK - 20209.
eGFR(mL/min/1.73m2) or CrCl (mL/min) ≥60 < 30a,b 30 to < 45a,b 45 to < 60a
References: 1. Invokana® Summary of Product Characteristics. Mundipharma 2020. 2. Dapagliflozin Summary of Product Characteristics. AstraZeneca 2019. 3. Empagliflozin Summary of Product Characteristics. Boehringer Ingelheim 2019. 4. Ertugliflozin Summary of Product Characteristics. MSD 2019.
Obesity in adults – a new clinical practice guideline from Obesity Canada
AUTHORS: Dr Cathy Breen, Clinical Specialist Dietitian in Obesity and Diabetes, St Columcille’s Hospital, Loughlinstown, Co Dublin
Obesity is a complex, and relapsing chronic disease underpinned by multiple genetic, metabolic, behavioural and environmental factors. Accumulation of excessive or abnormal adipose tissue can significantly impair health – type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), obstructive sleep apnoea, osteoarthritis, depression and gastroesophageal reflux disease are just a sample of some of its well documented complications. Consequently, individuals with obesity present for treatment to a wide variety of healthcare providers and services within the HSE. The recent publication of the ‘Canadian Adult Obesity Clinical Practice Guidelines’ are therefore a very welcome addition –providing an evidence- and experience-based framework for healthcare professionals looking to upskill in this important area.
Developed by Obesity Canada and the Canadian Association of Bariatric Physicians and Surgeons, the guidelines identify 80 key consensus recommendations, for healthcare providers, policy makers and people living with obesity, across a range of clinical and scientific areas. While a summary has been published in the Canadian Medical Association Journal, the 19 in-depth chapters on which the recommendations are based will remain an open-source, living document on the Obesity Canada website to be updated as new evidence emerges.
Reducing weight stigma in obesity management
One of the first chapters examines the challenge that weight stigma presents to delivering good obesity care in healthcare settings. Examples of weight stigma include the assumption that individuals with obesity are ‘non-compliant’ with medical advice and hence treatment strategies will not work, judgemental and rushed communication with healthcare professionals, lack of
appropriate equipment, and, in some cases, weight-based discrimination leading to patients being denied treatments. This section of the guideline outlines the negative physical, psychological and psychosocial consequences of weight stigma. Contrary to popular belief, weight stigma does not encourage positive behaviour change, and is instead associated with development of obesity, type 2 diabetes risk, increased cortisol, oxidative stress and C-reactive protein levels, eating disturbances, depression, anxiety and up to a 60 per cent increase in mortality risk. They recommend that all healthcare providers assess their own attitudes and beliefs regarding obesity (for example, using the online selfassessment tool the Implicit Association Test) and consider how these attitudes a ect their treatment of people with obesity.
Recommendations for reducing stigmatising consultations include avoiding judgemental words, images and practices and not making assumptions that all medical presentations in people with obesity are automatically related to body weight.
The science of obesity
One of the recommended strategies for reducing weight bias includes educating healthcare professionals about the nonmodifiable biological determinants of obesity, which is summarised in a fascinating chapter on the neurobiology of energy balance dysregulation in obesity. Appetite control is complex and involves the integration of hypothalamic control in the hypothalamus, hedonic control in the mesolimbic system and executive control exerted in the frontal lobe.
Hypothalamic control is primarily regulated in the arcuate nucleus hypothalamus.
Neurons co-ex-pressing AgRP and NPY stimulate hunger and trigger food-seeking behaviours, when activated by hormonal and
neural signals from the gut, adipose tissue and the peripheral organs. In contrast, a second set of neurons co-expressing POMC and CART, suppress food intake by firing through the downstream inhibitory Y1 and GABA receptors. Leptin and GLP-1 are two of the key hormones that mediate this homeostatic control of appetite and long-term energy reserve. As adiposity increases, circulating leptin levels increase and exert negative feedback to suppress appetite to prevent further weight gain. However, leptin resistance occurs in some people who have excessive adiposity, which can perpetuate the vicious cycle of fat mass accretion. Following weight loss in people with obesity, there is a cascade of neurohormonal adaptation, including a reduction in circulating anorexigenic hormones (leptin, insulin, GLP-1) and an increase in the orexigenic hormone ghrelin –all acting to encourage weight regain.
The emotional, pleasurable and rewarding aspects of eating associated with seeing, smelling or eating food - also known as hedonic eating – are regulated from the mesolimbic area. Signals transmitted by dopaminergic, opioid and endocannabinoid pathways mean that the brain can crave or enjoy food, even when an individual is completely satiated. Some people living with obesity may have a heightened anticipation of the pleasure of food driven by a dysregulation of dopamine, but a downgraded pleasure sensation on eating, leading to a drive to overeat. Controlling this dysregulation between wanting and liking with medications, hormonal regulation and cognitive behavioural therapy is a target for the treatment of obesity.
The cognitive lobe, responsible for executive functioning, overrides more primal behaviours driven by the mesolimbic system. While cognitive functioning works well under optimal conditions (rest, oxygen,
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 40
decreased stress, and supports) excess energy intake often occurs in the evening, during suboptimal conditions, following the accumulation of stressors and fatigue throughout the day. There may be other areas of executive dysfunction in some people living with obesity, primarily in response inhibition and cognitive flexibility, reducing conscious ability to manage eating behaviours.
Current research suggests that there is significant crosstalk between homeostatic and hedonic eating, as mediators such as leptin, insulin, ghrelin and GLP-1 also act on the dopaminergic neurons in the midbrain to modulate food reward and hedonic eating. This chapter also explores the genetics of obesity, adipose tissue inflammation and its role in the development of obesity complications, and the role of the gut microbiome.
Assessment of people living with obesity
While body mass index (BMI) has traditionally been used to define obesity, the new guidelines strongly recommend moving to a more comprehensive definition and assessment of individuals with obesity to guide better clinical care. BMI is a surrogate measure of adiposity and is associated with an increased risk of cardiovascular disease and all-cause mortality in the general population. However, individuals can experience good health at various BMI points, and defining obesity based on elevated BMI alone may lead to under- or over-diagnosis of obesity and contribute to weight bias and stigma. Defining obesity instead as ‘a chronic disease characterised by excessive or abnormal body fat that impairs health’, and using BMI only as a screening measure requiring further assessment is the more appropriate approach.
The Edmonton Obesity Staging System (EOSS) is the recommended framework to determine the severity of obesity and to guide clinical decision making. EOSS is a measure of the mental, metabolic and physical impact that obesity has had on the patients’ health and uses these factors
to determine their stage of obesity (from stage 0-4). It has been shown, in population studies, to be a better predictor of allcause mortality when compared to BMI alone. Recommendations for investigating metabolic and mechanical complications include measurement of blood pressure, fasting glucose or glycated haemoglobin, sleep disorders, osteoarthritis, nutritional deficiencies, lipid profile to determine cardio metabolic risk and, where appropriate, ALT to screen for NAFLD. Women with obesity and symptoms of PCOS should be screened for LH, FSH, total testosterone, DHEAS, prolactin, TSH and 17-hydroxyprogesterone levels. An obesity-centred history should include an assessment of weight history,
obesity and potential barriers to treatment with their patients.
Treatment options
A key tenant of the new guidelines is the recommendation that obesity treatment should focus on improving patient-centred health outcomes, not weight loss alone. Care plans should be individualised and address the root causes of obesity and provide support for both behavioural change (eg, nutrition, physical activity) and adjunctive therapies, which may include psychological, pharmacologic, and surgical interventions. The weight loss achieved with health behavioural changes is usually 3-5 per cent of body weight, which can result in meaningful improvement in obesity-related comorbidities but the amount of weight loss varies substantially among individuals, depending on biological and psychosocial factors and not simply on individual e ort.
nutrition history and eating patterns, physical activity and screen time, internalised weight bias, abuse, eating, mood and anxiety disorders, substance abuse, and screening for weight-promoting medications. The clinician conducting the assessment should also identify and document the patient’s values and goals around treatment and foster insight to help with long-term coping and selfmanagement skills.
The guideline document neatly tabulates the key elements of undertaking a comprehensive assessment using EOSS in five separate tables designed to support jobbing clinicians to identify root causes of weight gain as well as complications of
The weight at which the body stabilises when engaging in healthy behaviours can be referred to as the ‘best weight’; this may not be an ‘ideal’ weight on the BMI scale. Achieving an ‘ideal’ BMI may be very di cult. If further weight loss is needed to improve health and wellbeing beyond what can be achieved with behavioural modification, then more intensive pharmacological and surgical therapeutic options should be considered. Of note, it has generally been established that individuals with obesity who pursue treatment have expectations of weight loss that exceed what obesity management interventions are capable of achieving. These guidelines emphasise the benefits of collaborative, achievable, sustainable plans, which support patients to develop confidence to overcome barriers and fostering intrinsic motivation while impacting positively on health, function and quality-of-life, not just body weight.
Behavioural interventions, nutrition and activity
Individuals with obesity are often stigmatised and scrutinised for their food choices, portions and eating behaviours. Much of the social marketing e orts and public health and clinical messaging around
41 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
While body mass index (BMI) has traditionally been used to define obesity, the new guidelines strongly recommend moving to a more comprehensive definition and assessment of individuals with obesity to guide better clinical care
food and eating behaviours has focused on ‘eating less’. As a result of these messages, dieting and weight-loss focused outcomes perpetuate the notion that weight loss and/or ‘health’ can be achieved purely by caloric restriction, food deprivation and/ or ‘dieting’. These simplistic narratives neglect the evidence that weight loss may not be sustainable long-term, not because of personal choice or lack of willpower, but rather from strong biological or physiological mechanisms that protect the body against weight loss.
lipid profile, reduce cardiovascular events, reduce risk of type 2 diabetes and increase reversion of metabolic syndrome with little e ect on body weight and waist circumference.
Dietary approaches to stop hypertension (DASH), Nordic, vegetarian and low glycaemic index dietary patterns have been shown to reduce body weight and waist circumference, improve blood pressure, lipid profile, glycaemic control and reduce the risk of type 2 diabetes and coronary heart disease
Partial meal replacements (replacing one-to-two meals/day as part of a calorierestricted intervention) may reduce body weight, waist circumference, blood pressure and improve glycaemic control.
or obesity. Many exercise studies utilise the expertise of exercise professionals to guide patients and supervise exercise sessions.
Healthy eating is important, regardless of body size, weight or health conditions, and while some of the key messages from population level healthy eating guidelines can be used as a foundation for nutrition education, in the context of obesity management, the best nutrition approach is one an individual can maintain long-term to achieve health-related and/or weight-related outcomes. Nutrition interventions should be supported by a registered dietitian and use a shared decision-making approach to improve overall health, promote a healthy relationship with food, consider the social context of eating and promote eating behaviours that are sustainable and realistic for the individual. The following nutrition interventions could be considered:
Calorie-restricted dietary patterns (either intermittent or continuous) with variable macronutrient distribution ranges have been shown to achieve similar body weight reduction of ~5 per cent over six-12 months.
Mediterranean and portfolio dietary patterns may improve glycaemic control,
In addition, individuals with obesity are at increased risk for micronutrient deficiencies including but not limited to vitamin D, vitamin B12 and iron deficiencies. Restrictive eating patterns and obesity treatments (eg, medications, bariatric surgery) may also result in micronutrient deficiencies and malnutrition. Assessment including biochemical values should be undertaken to inform recommendations for food intake, vitamin/mineral supplements, and possible drug-nutrient interactions.
Physical activity should be encouraged, where possible, due to wide range of health benefits it brings across all obesity categories even in the absence of weight loss. Aerobic activity of 30-60 minutes on most days of the week has been shown to lead to a small amount of weight and fat loss, weight maintenance, and to a significant improvement in cardio-metabolic parameters, including glycaemia, blood pressure and lipid profile. In addition, resistance training (at least two days per week) may promote weight maintenance or modest increases in muscle mass or fat-free mass and mobility. In older adults especially, being physically active, attenuates the decrease in muscle and bone mass normally observed with diet alone. Regular activity can improve health-related quality-of-life, mood disorders (ie, depression, anxiety) and body image in adults with overweight
Multicomponent behavioural interventions with psychological components have been shown to be more e ective than nutrition or physical activity as stand-alone treatments. Combining behaviour modification (goal setting, self-monitoring and problem solving), cognitive therapy (reframing) and valuesbased strategies to alter nutrition and activity should be incorporated into care plans in obesity treatment to improve weight loss, health and quality-of-life. The Canadian guidelines recommend that healthcare providers should provide longitudinal care with consistent messaging to individuals with obesity to support the development of confidence in overcoming barriers (self-e cacy) and intrinsic motivation (personal, meaningful reasons to change), to encourage the patient to set and sequence health goals that are realistic and achievable and to analyse setbacks using problem-solving and adaptive thinking including clarifying and reflecting on values-based behaviours.
Pharmacotherapy
These guidelines recommend consideration of pharmacotherapy in individuals with a BMI ≥30kg/m2 or BMI ≥27kg/m2 with adiposity-related complications, and in conjunction with nutrition therapy, physical activity and psychological interventions. The three medications options reviewed are orlistat, liraglutide 3.0mg and naltrexone/ bupropion.
Orlistat (120mg three times daily) is a selective inhibitor of pancreatic lipase, inhibiting the breakdown of dietary triglycerides into absorbable free fatty acids, resulting in ~30 per cent of ingested triglycerides being excreted, creating a caloric deficit.
Liraglutide (a subcutaneously administered, human GLP-1 analogue) acts centrally on the POMC/CART neurons to improve satiation and satiety and reduce hunger, with a transient e ect to decrease gastric emptying.
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 42
The best nutrition approach is one an individual can maintain long-term to achieve health-related and/or weight-related outcomes
Naltrexone /bupropion is a centrally acting combination of two medications. Bupropion induces satiety by enhancing production and release of α-MSH and β-endorphin in the hypothalamus. Naltrexone disrupts the auto-inhibitory e ect of β-endorphin by blocking the µ-opioid receptors. Naltrexone/bupropion also influences the mesolimbic reward system to reduce cravings
Pharmacotherapy may be used to augment weight loss of 5-10 per cent and/or maintain weight loss that has been achieved by health behaviour changes, and to prevent weight regain. In addition, pharmacotherapy has been shown to prevent progression to and improve metabolic outcomes in type 2 diabetes, improve cardiovascular risk factors, NAFLD, PCOS, obstructive sleep apnoea and health-related quality-of-life.
The mechanism of action, adverse side e ects, safety, and tolerability of each agent must be considered in the context of each patient’s comorbidities and existing medications.
The cost of medications as well as the mode (oral versus subcutaneous) and frequency of administration can be a barrier to patient adherence and should be discussed. It is important to assess concomitant medications that a patient is taking as possible contributors to weight gain and to consider alternatives where appropriate. These guidelines recommend that if clinically significant weight loss is not achieved with pharmacotherapy, other factors contributing to perceived pharmacotherapy failure should be assessed, including inappropriate dosing or adherence, barriers to health behaviour change, and psychosocial or medical issues. They also note that there is considerable heterogeneity in the response to pharmacotherapy and consideration should be given to trying another agent if clinically significant obesity management success has not been achieved after three months on full/maximum tolerated dose and no other evident aetiologies of the lack of success are apparent.
Bariatric surgery
The sections on bariatric surgery are covered in three parts – patient selection and preop
workup; surgical options and outcomes; and postop management.
In general, bariatric surgery is indicated in patients with a BMI ≥40kg/m² or ≥35kg/ m² with at least one major obesity-related health complication. Similar to previous guidance in the area, these guidelines recommend that potential candidates for bariatric surgery undergo multidisciplinary evaluation and optimisation of their medical, mental, nutritional and functional health to assess their eligibility and safety to proceed with surgery. Potential candidates should be committed to engage in the educational process involved in preparing for bariatric surgery as well as understanding the necessity of long-term follow-up, both from a nutritional and medical perspective. Patients with unstable psychiatric illness, malignancy or other diseases associated with decreased life expectancy, substance abuse or an inability to adhere to long-term follow-up may be considered inappropriate candidates for surgery due to a high risk of short- and longterm complications. Because of the risks of post-operative complications associated with tobacco use, cessation prior to bariatric surgery is mandatory and should be maintained lifelong. Correction of nutritional deficiencies, as well as cardiopulmonary investigations, endoscopy and optimisation of glycaemia are also recommended pre-op.
The guidelines recommend that the decision regarding the type of surgery (sleeve gastrectomy, gastric bypass or duodenal switch) should be made in collaboration with a multidisciplinary team, balancing the patient’s expectations, medical conditions, and expected benefits and risks of the surgery. Overall, bariatric surgery has been shown to reduce long-term mortality, induce significantly better long-term weight loss compared to medical management alone, improve obesity complications including type 2 diabetes, dyslipidaemia, hypertension, and NAFLD, and to improve quality-of-life with obesity.
High quality post-operative care includes support and education on progression of diet, achieving protein targets, avoiding dyspepsia and managing alcohol intake.
Standard annual review should include assessment of nutritional intake, physical activity and behavioural strategies, compliance with multivitamin and mineral supplements, weight change, and biochemical investigation of potential nutritional deficiencies and obesity complications. Longer-term issues that may arise and require ongoing specialist care include gastrointestinal symptoms, nutritional issues, pregnancy management, psychological support, and weight regain. Consequently these guidelines recommend regular postop access to appropriate healthcare professionals until discharge is deemed appropriate for the patient.
Bringing it all together:
The 5 As of obesity management
A very helpful and practical element of these guidelines is the endorsement of a structured consultation framework for healthcare providers called the ‘5As of obesity management’.
ASKING for permission to discuss weight and explore readiness to engage in treatment.
ASSESSING obesity-related risks and root causes of obesity.
ADVISING on health risks and treatment options.
AGREEING on health outcomes and behavioural goals.
ASSISTING in accessing appropriate resources and providers.
This framework has been designed as a stepby-step guide for busy non-specialists who support patients in their practice to manage obesity, and has been shown to improve doctor-patient interactions when discussing this sensitive and complex topic.
In addition to the chapters summarised above, the live document also has sections on the epidemiology of obesity, mental health, commercial products and programmes, technology and virtual medicine and obesity management during the reproductive years in women. For more detail check out:
https://obesitycanada.ca/guidelines/chapters ■
References on request
43 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
Common thyroid disorders: An overview
AUTHOR: Theresa Lowry Lehnen, RGN, PhD, Post Grad Coronary Care, Clinical Nurse Specialist Practitioner, Associate Lecturer at Institute of Technology Carlow, and Member of the Irish Student Health Association (ISHA)
The thyroid gland is an endocrine organ, situated in the neck between the C5 and T1 vertebrae, anterior to the trachea and inferior to the larynx. It plays a major role in the metabolism, growth and development of the human body. The thyroid gland regulates a wide range of physiological functions in the body via the secretion of thyroid hormone (TH). Its hormones regulate basal metabolism, oxygen use, nutrient metabolism, the production of ATP, and calcium homeostasis. They also contribute to protein synthesis and the normal growth and development of body tissues, including maturation of the nervous system, and increase the body’s sensitivity to catecholamines.

Wing-shaped left and right thyroid lobes flank the medial region, called the isthmus. The parathyroid glands, responsible for the production of PTH, which control calcium levels in the body are situated on the posterior surfaces of the thyroid lobes. The functional unit of the thyroid gland is the follicle, a roughly spherical group of cells arranged around a protein-rich storage material called colloid, which is the centre of thyroid hormone production. Hormones are produced in the colloid when atoms of the mineral iodine attach to a glycoprotein, called thyroglobulin that is secreted into the colloid by the follicle cells.
The thyroid gland produces TH which includes levothyroxine (T4) and triiodothyronine (T3), under the direct control of thyroid-stimulating hormone (TSH), secreted from the pituitary gland. TSH is under the control of thyrotropinreleasing hormone (TRH), secreted by the hypothalamus. Regulation of thyroid function depends on normal functioning of the hypothalamic-pituitary-thyroid axis via a negative feedback loop, whereby precise control of the axis is maintained
by the inhibitory actions of T4 and T3 on both TRH and TSH secretion. Serum TSH is sensitive to small changes in serum TH concentrations. A 50 per cent reduction in free T4 produces a 90-fold increase in serum TSH. T3 is the active form of TH and has a shorter half-life (t1/2 =1 day) in the circulation compared with T4 (t1/2 =7 days). Most of the T3 in the body results from de-iodination of T4 in the peripheral tissues. More than 99 per cent of circulating TH is bound to protein, primarily thyroid-binding globulin (TBG). The levels of free circulating
TH remain normal irrespective of the concentration of binding proteins. This is important for interpretation of thyroid function tests.
Hypothyroidism
Hypothyroidism, commonly known as an underactive thyroid, is an endocrine disorder caused by decreased thyroid function. It is one of the most common conditions seen in clinical practice.
Hypothyroidism often results from dysfunction of the thyroid itself (primary hypothyroidism) but can also occur because
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 44
FIGURE 1: Thyroid gland physiology Source: https://opentextbc.ca/anatomyandphysiology/chapter/17-4-the-thyroid-gland/
of defects along the hypothalamic/ pituitary axis or from intake of lowerthan-required doses of exogenous thyroid hormone in patients with primary hypothyroidism.
Hypothyroidism is more common in women and in people over the age of 60 years. It is among the most frequent chronic diseases in the elderly, and levothyroxine (l-T4) is within the 10 most prescribed medications worldwide in the general population. In Ireland, levothyroxine is one of the most frequently prescribed medicines on the community drug schemes, reflecting a high level of hypothyroidism in clinical practice.
Causes
The thyroid gland is the main site of iodine uptake in the body, which is incorporated into thyroid hormone. A deficiency of iodine in the diet is the most common cause of hypothyroidism worldwide. In countries with enough iodine in the diet, the most common cause of hypothyroidism is the autoimmune condition Hashimoto’s thyroiditis. Less common causes include radioactive iodine injury to the hypothalamus or the anterior pituitary gland, certain medications, for example lithium, a congenital nonfunctioning thyroid at birth, or previous thyroid surgery.
The recommended daily intake of iodine for adults is 150-300mcg. The highest dietary iodine content is found in fish, with smaller amounts in milk, eggs and meat. Intake of less than 50mcg/day is associated with reduced thyroid function, either hypothyroidism in adults, cretinism in the presence of inadequate intake from birth, or goitre whereby the gland size increases to compensate for the lower iodine in an e ort to maintain normal TH levels. Because thyroid hormone plays
in pleural or pericardial e usions, or even rhabdomyolysis.
Diagnosis
Blood tests measuring TSH and thyroxine levels are required to make a diagnosis of hypothyroidism. The normal reference range for TSH is 0.4-4.0mU/L, however, the range may vary according to assay and laboratory. An elevated TSH level and a low T4 usually diagnose hypothyroidism. Free T4 and sometimes free T3 levels may be required if the diagnosis is uncertain. The presence of specific anti-thyroid antibodies confirms autoimmune thyroiditis, but may not always be found.
Treatment
a fundamental role in the regulation of normal metabolism, reduced thyroid hormone levels in hypothyroidism are associated with metabolic slowing leading to an array of signs and symptoms, including fatigue, reduced exercise capacity, muscle weakness, weight gain, bradycardia, cold intolerance, slowing of the normal reflex relaxation phase, constipation, depression, and menstrual irregularities. In severe cases, hypothyroidism can result
SIGNS SYMPTOMS
Eyebrow and hair thinning (C)
Skin coarsening (C)
Fatigue
Weight gain
Tendon relaxation phase slowing (C)Cold intolerance
Facial/periorbital oedema (O)
Macroglossia (O)
Bradycardia (O)
Pericardial e usion (R)
Pleural e usion (R)
Rhabdomyolysis (R)
Mental slowing
Muscle weakness
Reduced exercise capacity
Constipation
Xeroderma
Depression, menstrual irregularities
C = COMMON O = OCCASIONAL R = RARE
In most patients with hypothyroidism, the condition is permanent and requires thyroid hormone replacement therapy for life, with a goal of maintaining euthyroidism (normal thyroid gland function). Treatment of hypothyroidism is with the synthetic prohormone levothyroxine. T4 is the replacement therapy of choice because of its long half-life, once-daily administration, and low cost. Because levothyroxine has a biological half-life of approximately one week, once-daily dosing gives near steady-state concentrations of T4 and T3. Full thyroid hormone replacement with levothyroxine is typically 1.6µg/kg of total body weight provided daily, although in patients with significant obesity, lean body mass may need to be considered for initial calculations to avoid overtreatment.
The starting dosage depends on the age, clinical status and TSH level of the patient. For patients aged under 50 years, with no evidence of cardiovascular disease (CVD), a starting dose of 50-100mcg/day of T4 is recommended. In older patients and those with CVD the starting dose should not exceed 50mcg. In the presence of severe cardiovascular disease, this may be further reduced to 25mcg. Response to T4 therapy is monitored by TSH levels, taking into account the time lag in TSH response and the dosage increased at 2550mcg increments, up to a maximum daily
45 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
TABLE 1: Signs and symptoms of hypothyroidism
Hypothyroidism is more common in women and in people over the age of 60 years
Source: Drake, M. (2018). Hypothyroidism in Clinical Practice. Mayo Clinic Proceedings. Volume 93, Issue 9, 1169–1172.
dose of 200mcg, until clinical evidence of normal function is present and TSH levels are restored to within the normal range. T4 replacement therapy is for life except in the case of post-viral or postpartum thyroiditis. T4 should be taken on an empty stomach in the morning. It should not be taken at the same time as certain minerals or vitamins that interfere with its absorption such as calcium. Microsomalinducing enzymes such as rifampicin and carbamazepine enhance metabolism. The dosage of concomitant medications, eg, for CVD, diabetes mellitus and anticoagulation may need to be readjusted when normal thyroid function is restored. Side e ects are usually due to excessive dosage for the individual and include gastrointestinal (GI) disorders, palpitations and myalgia. These are reversible upon reduction of the dosage.
Most patients with symptomatic hypothyroidism begin to notice an improvement within several weeks of commencing treatment, although resolution of symptoms once maintained on a stable dose may not occur for several months. If a patient continues to have clinical symptoms despite treatment and return of TSH levels to within the normal range, certain factors should be considered. Sub-optimal compliance or problems with absorption will require further investigation. Where the TSH is just below the upper level of normal, but the patient is still clinically hypothyroid, T4 dosage adjusted to achieve TSH levels at the lower level of normal is recommended. Concomitant illness such as depression, often associated with Hashimoto’s thyroiditis, may occur and need to be treated independently. Occasionally a patient may require both T4 and T3 replacement in order to restore physiological function, however, routine use of combination T4 and T3 therapy is not recommended as its benefit have not been confirmed by clinical studies.
Hyperthyroidism
Hyperthyroidism or an overactive thyroid occurs when the thyroid gland produces too much of the hormone thyroxine.
Hyperthyroidism can accelerate the body’s metabolism, causing unintentional weight loss and a rapid or irregular heartbeat. Common symptoms of an overactive thyroid include nervousness, anxiety and irritability, hyperactivity, mood swings, fatigue, di culty sleeping, sensitivity to heat, muscle weakness, diarrhoea, frequent urination, persistent thirst, itchiness and loss of libido. Signs of hyperthyroidism include a goitre, a rapid resting heart rate, uncoordinated heart rhythm, tremor, warm, moist skin, redness on the palms of the hands, loosening of nails in the nail beds, urticaria, alopecia, and twitching.
common cause of hyperthyroidism after Graves’ disease, and occurs when there are two or more nodules in the thyroid gland. Solitary thyroid adenoma (single nodule) account for about 5 per cent of cases of hyperthyroidism.
Occasionally a viral infection can a ect the thyroid gland causing thyroiditis. Thyroiditis is associated with transient over-activity, which can last for a few weeks before the thyroid function changes and becomes underactive (hypothyroidism) before finally returning to normal thyroid function. Post-partum thyroiditis can also occur. Other uncommon causes include iodine-containing drugs, such as amiodarone and iodine-induced hyperthyroidism, sometimes referred to as Jod-Basedow phenomenon.
Symptoms
Causes
Graves’ disease, an autoimmune condition, is the most common cause of hyperthyroidism, and accounts for 80 per cent of hyperthyroidism in iodine-replete areas. It is due to the production of antiTSH receptor antibodies that stimulate the thyroid gland, resulting in overproduction of TH. The female male ratio varies from 5-10:1 and the peak incidence is between 40 and 60 years of age although it can occur at any age. It can run in families and is more common in smokers. Ocular symptoms diagnostic of Graves’ disease include proptosis, extra-ocular muscle problems, diplopia, corneal problems due to lid closure failure, and sight loss due to optic nerve compression.
The next most frequently occurring cause of hyperthyroidism is autonomous overproduction of TH by either a solitary thyroid adenoma or multinodular goitre. Nodules that contain abnormal thyroid tissue are described as toxic. Toxic multinodular goitre is the second most
Signs and symptoms of hyperthyroidism, irrespective of the cause, relate primarily to over-stimulation of the cardiovascular, gastrointestinal and nervous systems. Younger people with thyrotoxicosis may present with any of the symptoms but older individuals are more likely to present with cardiovascular disease such as atrial fibrillation and heart failure. Although all patients can present with ocular symptoms, certain ocular signs and symptoms are diagnostic of Graves’ disease. More than 80 per cent of patients with Graves’ disease are noted to have eye involvement on orbital imaging, although this is clinically apparent in only 30-50 per cent of patients. Any history of interference with vision requires emergency referral to specialist services. Untreated hyperthyroidism may lead to CVD and severe thyrotoxicosis, known as thyroid storm. This very rare condition, usually precipitated by infection is associated with a mortality of 20-50 per cent.
Diagnosis
A diagnosis of hyperthyroidism is confirmed by physical examination and blood tests. If TSH is below the reference range and the thyroxine (FT4) above the reference range this usually indicates an overactive thyroid. Free T3 levels may be
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 46
Graves’ disease, an autoimmune condition, is the most common cause of hyperthyroidism
helpful to confirm the diagnosis, especially if T3 toxicosis is suspected with normal T4. The presence of anti-TSH receptor antibodies is diagnostic of Graves’ disease and the presence of other anti-thyroid antibodies may be helpful in diagnosing autoimmune thyroiditis.
Treatment
Treatment options for hyperthyroidism include pharmacotherapy, surgery and radioactive iodine. Treatment is tailored to the individual patient, taking into account the likelihood of remission with medication alone, goitre size, presence of co-morbidities, patient preference and the potential of future pregnancies for females. Individuals with confirmed hyperthyroidism should be referred for specialist care in order to optimise their management plan.
Antithyroid drugs, known as thionamides, inhibit the production of TH in the thyroid gland, by interfering with the iodination process. They may have immunosuppressive e ects, which can be beneficial in promoting remission in autoimmune hyperthyroidism. Carbimazole is usually commenced at a daily dose of 20-60mg, titrated against thyroid function (TH/TSH), until the patient is euthyroid (usually by week four). Maintenance therapy (usual range 5-15mg/day) is continued for up to 18 months according to TH/TSH response. Common side e ects include skin rash, pruritus, arthralgia, and GI upset which are usually self-limiting. A rare adverse drug reaction is agranulocytosis. A full blood count should be checked regularly, especially in the early months of treatment and patients should be advised to seek immediate attention if they develop a sore throat, fever or other symptoms of infection. Cholestatic jaundice can also be a rare side e ect.
In addition, patients may require a β-blocker to alleviate tremor, palpitations and sweating, while thionamides take e ect. Atrial fibrillation should revert once normal thyroid function has returned. However, if atrial fibrillation is still present
at 12 weeks, it is unlikely to reverse and the need for anticoagulation should be considered. Patients with ophthalmic Graves’ disease require a specialist review.
Subclinical thyroid dysfunction
Subclinical thyroid diseases are characterised by abnormal serum TSH levels associated with normal thyroid hormone concentrations.
Subclinical hyperthyroidism is defined as low serum TSH but normal free T4 and T3, in the absence of hypothalamic or pituitary disease, non-thyroidal illness or medications that inhibit TSH secretion such as glucocorticoids or dopamine. The causes of subclinical hyperthyroidism are divided into two subgroups: exogenous and endogenous. Exogenous subclinical hyperthyroidism results from thyroid hormone replacement therapy. The endogenous causes of subclinical hyperthyroidism are similar to causes of overt hyperthyroidism. Graves’ disease is the most common cause in young people, whereas toxic nodular goitre, solitary or multinodular, is more common in the elderly.
Subclinical hypothyroidism is defined as a raised serum TSH above the reference range with a normal free T4 and T3. It is the commonest type of subclinical thyroid dysfunction, and is most often due to chronic autoimmune thyroiditis. Subclinical hypothyroidism increases with age and a ects up to 18 per cent of the elderly, with a higher prevalence in women than men. Data has shown an increased risk of coronary heart disease, heart failure, and cardiovascular mortality among a ected adults. Conflicting results have been found with the association between subclinical hypothyroidism and cognitive impairment, depression and the risk of fractures. Management strategies including screening and treatment of subclinical hypothyroidism are still controversial.
Goitre
A goitre usually presents as a palpable or visible enlargement of the thyroid gland at the base of the front of the neck. Goitres
can have a number of causes including hypothyroidism, hyperthyroidism, pregnancy, lack of iodine, a growth, cyst, or tumour in the thyroid gland. If associated with hypothyroidism or hyperthyroidism, a goitre may present with symptoms of the underlying disorder. The size of a goitre can vary from person to person. In most cases the swelling is small and does not cause any symptoms. In more severe cases, symptoms may include coughing, tightness in the throat, changes to the voice such as hoarseness, dysphagia and di culty breathing.
Goitres are classified as nodular or di use. Nodular goitres can be uninodular or multinodular. Uninodular goitres can be inactive, or active (toxic) producing thyroid hormone. Multinodular goitre can also be inactive or toxic. Toxic multinodular goitre is associated with hyperthyroidism. The nodules grow at varying rates and secrete thyroid hormone autonomously, suppressing TSH-dependent growth and function in the rest of the gland. Inactive nodules in the same goitre can be malignant. Di use goitre is where the whole thyroid appears enlarged due to hyperplasia. Goitres are classified according to their size. Grade 0: No goitre is palpable or visible. Grade 1: Palpable goitre, but not visible when the neck is held in a normal position. Grade 2: A swollen neck consistent with a goitre on palpation and which is visible when the neck is held in the normal position. Treatment for goitre depends on the underlying cause and can include medication such as thionamides for hyperthyroidism and synthetic hormone levothyroxine for hypothyroidism. Other treatments can include iodine supplements, radioiodine treatment and thyroid surgery. If the goitre is small, it may require no immediate treatment only monitoring for changes.
Thyroid cancer
Although thyroid cancer is rare, it is still the most common form of endocrine cancer.
About 162 people are diagnosed with thyroid cancer each year in Ireland – 117 women and 45 men (Irish Cancer Society,
47 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
2014). Thyroid cancer can now be diagnosed early due to better diagnostic techniques. The most common thyroid cancers are di erentiated and include papillary and follicular cancers. Papillary carcinoma is the most common thyroid cancer. It is more common in younger people, particularly women. Follicular carcinoma is less common and tends to occur in slightly older people. These types of cancer are usually treated with surgery and radioactive iodine treatment (RAI).
Prognosis for patients with di erentiated thyroid cancer is very good, with an 80-90 per cent cure rate. Most can be treated successfully with surgery, radioactive iodine or a combination of both. Other less common types of thyroid cancers are medullary, anaplastic, and lymphoma. Medullary carcinoma is a rare cancer that is sometimes hereditary. Most thyroid cancers are treatable and curable, but it is possible that they will recur, so lifelong follow-up is very important.
Thyroid dysfunction and pregnancy
Pregnancy can greatly a ect the functioning of the thyroid gland and is associated with a 10-40 per cent increase in the size of the gland, a 50 per cent increase in the production of thyroxine (T4) and (T3), and a 50 per cent increase in the daily requirement of iodine. These physiological changes can render a pregnant, iodinedeficient, euthyroid woman in the first trimester hypothyroid during the later stages of pregnancy.
Hypothyroidism can make conception di cult, however, it should be suspected in pregnant women with vitiligo, premature grey hair or a positive family history. Pregnant women already on T4 replacement therapy will require increased dosage during pregnancy to achieve normal TSH levels.
The prevalence of hypothyroidism during pregnancy is estimated to be 0.3-0.5 per cent for overt hypothyroidism and 2-to-3 per cent for subclinical hypothyroidism (SCH). Thyroid hormone is critical for
foetal brain development. In the first trimester of pregnancy the foetus is completely dependent on the mother for the production of thyroid hormone. The foetus starts to produce its own thyroid hormones from 16-to-20 weeks of pregnancy, but it still requires the mother to ingest adequate amounts of iodine to aid production of thyroxine.
hyperthyroidism is the most common cause, accounting for 1-to-3 per cent of cases of hyperthyroidism, and is characterised by transient hyperthyroidism, limited to the first half of pregnancy characterised by elevated fT4 or adjusted T4 and suppressed or undetectable serum TSH, in the absence of serum markers of thyroid autoimmunity. It is thought to occur because of high hCG levels and may be associated with hyperemesis gravidarum, multiple gestation, hydatidiform mole, or choriocarcinoma. Grave’s disease is the second most common cause of hyperthyroidism, accounting for 0.1-1 per cent of cases and may be diagnosed for the first time during pregnancy or be present as a recurrent episode. Other causes include toxic multinodular goitre, toxic adenoma, and factitious thyrotoxicosis.
Untreated hypothyroidism is associated with complications in pregnancy, such as spontaneous miscarriage, maternal anaemia, pre-eclampsia, small for gestation age babies, pre-term birth and postpartum haemorrhage. Levothyroxine requirements in pregnancy frequently increase by 25-30 per cent and in some women by 50 per cent. TSH and free T4 levels should be checked once a pregnancy is confirmed. TFT’s should be monitored every four-tosix weeks until levels become normal and trimester specific ranges should be used when testing.
It is important to remind patients not to take mineral or vitamin supplements at the same time as their T4 medication. Antenatal vitamin supplements containing iron and calcium can impair the absorption of thyroid hormone from the GI tract. Thyroxin should also be taken either one hour before or two hours after food. Postpartum the woman can usually return to her pre-pregnancy dose of levothyroxine. TFTs should be checked at the six-week post-natal appointment.
Hyperthyroidism can complicate pregnancies, although its incidence is less than hypothyroidism. Gestational
Postpartum thyroiditis is the occurrence, in the first postpartum year, of thyroid dysfunction in previously euthyroid women. Postpartum thyroiditis is characterised by transient thyrotoxicosis followed by transient hypothyroidism, with an eventual return to a euthyroid state by the end of the first postpartum year. The initial thyrotoxic phase usually occurs three months after delivery of the baby and is characterised by nervousness, irritability, and palpitations. These symptoms are often falsely attributed to anxiety neurosis occurring after childbirth. Postpartum thyroiditis is an exacerbation of an underlying autoimmune thyroiditis, aggravated by the immunological rebound that follows the partial immunosuppression of pregnancy. It is associated with the presence of antithyroid antibodies in the first trimester, with increased titres conferring an increased likelihood. Women with other autoimmune disorders, such as type 1 diabetes and systemic lupus erythematosus, also have an increased risk. There is a 70 per cent recurrence rate of postpartum thyroiditis in subsequent pregnancies amongst individuals who recover. ■
References on request
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 48
Although thyroid cancer is rare, it is still the most common form of endocrine cancer
Managing polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age and a major cause of infertility
PCOS is common, a ecting an estimated one-in-10 women. It can cause period problems, reduced fertility, excess hair growth and acne. Many women with PCOS are also overweight.
The aetiology of PCOS remains unknown. Increased frequency of hypothalamic release of gonadotropin-releasing hormone (GnRH) is found in women with PCOS. It is unclear if this defect in GnRH pulse secretion is a primary or secondary abnormality.
Increased frequency of GnRH favours the secretion of luteinising hormone (LH) versus follicle stimulating hormone (FSH) from the anterior pituitary so that LH pulses also increase in frequency and amplitude.
The ovary in PCOS responds to the LH stimulation with a preferential increase in the production of androgens versus oestrogen. Low circulating progesterone as a result of oligo-ovulation and elevated androgen levels create a feedback that further potentiates inappropriate hypothalamic pituitary gonadotropin secretion and leads to a vicious cycle.
Oestradiol levels are typically normalto-low, but oestrone levels are typically elevated. This is because of conversion of androstenedione to oestrone in adipose tissue, which further stimulates LH and spreads FSH.
Diagnosis
PCOS is a diagnosis of exclusion and other treatable conditions also with hyperandrogenism should be excluded, such as Cushing’s syndrome, androgenproducing tumours, and non-classical
congenital adrenal hyperplasia.
The minimum criteria to diagnose PCOS are two of the three following:
▸ Anovulation.
▸ Clinical or biochemical evidence of hyperandrogenism.
▸ Polycystic ovaries.
The specific ultrasound characteristics are presence of 1.2 follicles in each ovary measuring 2-to-9mm in diameter and increased ovarian volume and stoma. Only one ovary with these criteria is su cient.
These ultrasound criteria do not apply to women on oral contraceptives.
If there is evidence of a dominant follicle, the scan should be repeated.
Pathophysiology
Evidence suggests that PCOS may be hereditary, but the pathogenesis of the syndrome points to a complex multigenic disorder.
Candidate genes that may be responsible for alterations in ovarian, hypothalamic and
LIFESTYLE CHANGES FOR PCOS
Weight loss
If overweight, even a small amount of weight loss can improve PCOS symptoms. Between 5-to-10 per cent loss of total body weight can help normalise menstrual cycles, regulate blood sugar and increase a woman’s chances of conceiving. A weight loss of between 1-to-2lbs (1/2-to-1kg) a week is a safe and practical target. However, a healthy diet is bene cial to all women with PCOS, even if not overweight.
Eat regular meals
This will help to keep insulin levels stable throughout the day. Eating often can also help to control appetite. Preplanning meals is also a key component of this.
Eat a balanced diet
This includes eating lots
of fruit and vegetables, choosing low-fat dairy foods and lean meats and sh. It is also critical to limit the amount of fatty, sugary and processed foods and drinks consumed.
Vitamin D
There have been a number of studies suggesting that a lack of vitamin D leads to insulin resistance. Supplementing with vitamin D may help to prevent this, however, more research is needed before this can be con rmed.
Limit added sugar
It is critical to avoid highsugar foods with PCOS. Eating less sugar results in lower blood glucose levels. This decreases insulin levels and reduces male hormone levels. Most women with PCOS crave sugary foods, even after
eating meals. This is due to increases in insulin.
Stress
Managing stress levels is an important part of dealing with PCOS. Stress can worsen PCOS symptoms by increasing testosterone, which can increase insulin resistance.
Exercise
Exercise is also a great way of reducing stress levels and simultaneously targeting weight. There are many bene ts to be gained from being physically active, including improving insulin resistance. At least 30 minutes of moderateintensity physical activity most days of the week is advised. For weight loss, additional physical activity may be needed.
49 Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology
Source: Irish Nutrition and Dietetics Institute (INDI) www.indi.ie
AUTHOR: Dr Mary Ryan, Consultant Endocrinologist, Bon Secours Hospital Limerick at Barringtons
insulin receptor function have been the focus of linkage and case control studies.
It has also been suggested that prenatal androgenisation of the female foetus induced by genetic and environmental factors, or the interaction of both, may programme di erentiating target tissues towards the development of PCOS phenotype in adult life.
Conditions associated with PCOS include the metabolic syndrome, glucose intolerance or type 2 diabetes, dyslipidaemia, hypertension, sleep apnoea and infertility.
The chronic oestrogenic stimulation seen in PCOS results in endometrial hyperplasia and dysfunctional uterine bleeding and may lead to endometrial carcinoma.
WOMEN WITH POLYCYSTIC OVARY SYNDROME FACE HIGHER RISK OF BREATHING DIFFICULTIES
Priscilla Lynch
Women with PCOS are more likely to develop poor respiratory health based on lung function tests, according to research presented at the 2019 European Respiratory Society International Congress.
The study suggests that PCOS may also be responsible for lower lung capacity in some women, which may lead to shortness of breath.
The research was presented by Dr Diana van der Plaat, a RESPIRE 3 fellow based at the National Heart and Lung Institute, Imperial College London, UK. She said: “We tend to think of lung diseases, like chronic obstructive pulmonary disease (COPD), as being more common in men and in smokers. But a striking proportion of COPD patients have never smoked. In recent years, there has been an increase in lung disease and deaths from lung disease in women, and we want to understand why.
“This work is part of a big study looking at hormonal factors and lung health in women. PCOS is very common so it’s important to nd out whether this condition is associated with poor lung health.”
Researchers used Mendelian randomisation to investigate whether PCOS may lead to lower lung function, examining lung function data from 182,619 women from the UK Biobank project. They also used previously published genetic data on PCOS from several worldwide studies.
In each woman, lung function was tested with a spirometer. Researchers used genetic variants associated with PCOS to investigate
whether PCOS can cause poor respiratory health based on these lung function tests.
The results suggest that women with PCOS were around 10 per cent more likely to have lower lung function, compared to other women.
Dr van der Plaat explained: “We use genetic variants as a proxy for PCOS because these genetic variants are there from the moment a person is conceived, and they do not change over the life course. This means that we can investigate the causal relationship between PCOS and lung function and show that the trend we see in women’s breathing is likely to be caused by PCOS, rather than the other way around.
“We found that women with PCOS have a small increased risk of having impaired lung function. Poor lung function can cause di culty in breathing and inadequate exchange of oxygen to the blood or carbon dioxide from the blood. This research highlights the fact that PCOS can a ect di erent parts of a woman’s body, not only her reproductive organs. We need to do more research to understand why women with PCOS also have poorer lung health.”
While the study does not explain why PCOS and lung function might be linked, the connection may relate to insulin levels and diabetes. Women with PCOS are known to face a higher risk of diabetes and this in turn has been linked with lower lung function.
Treatment
The first-line treatment for overweight and obese patients with PCOS should be weight loss and lifestyle modification.
The mainstay of treatment of oligomenorrhea/amenorrhea includes a combination oral contraceptive preparation that contains low-dose ethinyl estradiol, in conjunction with a progestin with low androgenic activity or even antiandrogenic activity.
An anti-androgen may be added for additional benefit in controlling hirsutism when this is the main complaint.
Insulin sensitisers can reduce insulin and androgen levels and increase ovulatory cycles.
Clomiphene citrate has been shown to be superior to metformin in achieving ovulation but has a risk of multiple pregnancies. Combinations of clomiphene with metformin may increase pregnancy.
If pregnancy is not achieved, consideration can be made for the use of injectable gonadotropins or in vitro fertilisation (IVF).
Comorbidities
There needs to be a greater public awareness that patients with PCOS are at high risk of developing gestational diabetes, pregnancyinduced hypertension, pre-eclampsia, and preterm labour. Studies have shown that a longer or irregular menstrual cycle length can lead to a two-fold increase in the risk of hypertension in women with PCOS. The prevalence of hypertension increases dramatically by the time of peri-menopause. Women with PCOS also have an increased risk of dyslipidaemia and have been shown to have abnormal vascular function, higher rates of coagulopathy and increased markers of inflammation. Whether women with PCOS and metabolic syndrome are at increased cardiovascular risk remains a question for future investigations, although predictions of a seven-fold relative risk for myocardial infarction have been made. Sleep apnoea also occurs at a higher incidence with PCOS. ■
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 50
Vitreous haemorrhage in a diabetic patient with retinal venous loops and pre-proliferative diabetic retinopathy
CASE REPORT
A 64-year-old female attended the diabetic retina treatment clinic in February 2020 for a routine fundus examination. Her medical history is signi cant for poorly controlled type 2 diabetes necessitating insulin (known duration of 15 years), hypertension and dyslipidaemia. She has a history of bilateral background diabetic retinopathy (DR) and bilateral diabetic macular oedema treated with a course of intravitreal bevacizumab in 2017.
On examination her best corrected visual acuity (BCVA) was 6/9 in the right eye and 6/6 in the left eye. The anterior segment exam was unremarkable except for bilateral early lens opacities. Dilated fundus examination was notable for preretinal haemorrhage inferior to the macula in the left eye. The haemorrhage is emerging from an omega shaped venous loop nasal to optic nerve. Another venous loop was noted on the superotemporal arcade (see Figure 1). Optical coherence tomography (OCT) showed slight increase in her centre, involving clinically signi cant macular oedema (ciCSMO) in both eyes. Fundus uorescein angiography (FFA) showed staining and late leakage of the nasal venous loop wall and nonperfusion of the surrounding capillary bed without signs of progressive leakage from neovascularisation, (see Figures 2, 3). Also of note is the macular ischemia with enlargement of the foveal avascular zone and multiple areas of capillary non perfusion in the mid periphery and peripheral retina.
Retinal venous loops (RVL) are rare manifestations of diabetic retinopathy usually identified by fundoscopy or colour fundus photography. Although they are considered one of the venous morphological changes during the course of diabetic retinopathy, their prevalence and its predictive value reported in the literature may be underestimated due to the focus on other vascular. Diabetic retinopathy venous abnormalities such as beading, loops, reduplications, and shunts indicate that retinopathy has entered a pre-proliferative stage.1 Vitreous haemorrhage in the context of venous
loops could occur due to proliferative changes at areas of nonperfusion or due to posterior vitreous traction on the loop itself.2 In this case report article wereport vitreous haemorrhage in the setting of retinal venous loops and pre-proliferative retinal changes.

The patient was listed for scatter panretinal photocoagulation (PRP) and a first session was performed using Valon laser with 2061 pattern spots being delivered. At the time, the BCVA in the left eye was 6/7.5 and the fundus exam noted significant improvement of the pre-retinal haemorrhage.
Two weeks post-laser treatment the patient presented with sudden and severe decrease in vision. On ocular examination, BCVA in the left eye was 5/60 and the fundus exam showed an important worsening of the preretinal bleeding, covering the inferior half of the macula (Figure 4).
Fill in PRP with 700 single spots was done on the day and the patient was seen within three weeks, when good improvement was noted (BCVA in left eye 6/48), but the subhyaloid haemorrhage settling inferiorly (Figure 5).
At the time, OCT showed ciCSMO in both eyes and the patient was started on a course of intravitreal bevacizumab. However,
51 Endocrinology and Diabetology | Volume 6 | Issue 8 | 2020
AUTHORS: Dr Rashid Nahar, Dr Cosmina Barac, and Prof David Keegan, Diabetic Retinal Treatment Centre, Department of Ophthalmology, Mater Misericordiae University Hospital, Dublin
In this case report article we report vitreous haemorrhage in the setting of retinal venous loops and preproliferative retinal changes
FIGURE 1: Coloured wide eld fundus images right and left eye
during the course of injections, a dense vitreous haemorrhage occurred in the left eye, without any view of the fundus. The patient was listed for pars plana vitrectomy with endolaser panretinal photocoagulation to treat the non-clearing vitreous haemorrhage. During the surgery, after clearance of the haemorrhage, the retina was inspected. The loops were confirmed and no eccentric areas of neovascularization were noted. Laser was applied based on the presence of haemorrhage and extensive nonperfusion noted on prior angiograms.


Discussion
It has been shown that diabetic patients
displaying RVL and reduplications have a duration of diabetes similar to that of patients with proliferative diabetic retinopathy, and that new vessels may even originate from the venous abnormalities. Venous loops and reduplications are rarer than venous beading and might result from accentuation of a bead, traction from vitreoretinal adhesions or may be shunt vessels that bypass a localised occlusion of a larger retinal vein.1,2,5 Changes in vessel calibre are caused by regulatory responses of the vascular, smooth muscle adjacent to areas of nonperfusion because of the lack of sympathetic vasomotor innervation in retinal vessels, reflecting increased retinal ischaemia. Venous beading in diabetic retinopathy has traditionally been known to indicate future progression of non-proliferative retinopathy to proliferative diabetic retinopathy,3 whereas some evidence showed that the presence of loops and reduplications indicates established proliferative diabetic retinopathy.4
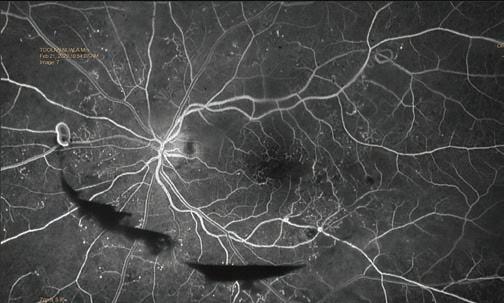
Conclusion
Although venous loops do not appear to lead to sight-threatening changes in the diseased retina, further understanding of the pathogenesis of retinal venous loops is needed to establish the relative weighing of this criterion in diagnosing severe non-proliferative retinopathy/proliferative retinopathy. We propose that careful observation of features of non-proliferative diabetic retinopathy like venous loops lesions may help to stratify people based on the likelihood of rapid progression whether from the onset of proliferative
disease or from risk of bleeding due to vitreous traction. This will be the focus of upcoming analysis from our department.
Consent
A written informed consent was obtained from the patient to publish medical data and figures described in this case report.
Conflict of interest
The authors report no conflicts of interest. The authors declared no potential conflicts of interest with respect to this case report. ■
References
1. Bek, T. (1999), Venous loops and reduplications in diabetic retinopathy Prevalence, distribution, and pattern of development. Acta Ophthalmologica Scandinavica, 77: 130-134. doi:10.1034/j.16000420.1999.770202.x
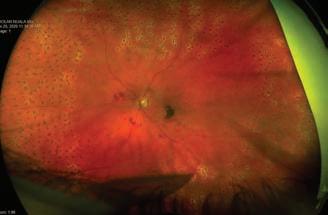
2. Mathis, A, et al. “[Hemorrhage in the Vitreous and Retinal Venous Loop in a Diabetic Subject].” Journal Francais D’ophtalmologie, vol. 7, no. 3, 1984, pp. 201-3
3. Wong TY. Retinal vessel diameter as a clinical predictor of diabetic retinopathy progression: time to take out the measuring tape. Arch Ophthalmol.2011; 129: 95–96
4. Sato Y, Kamata A, Matsui M. Clinical study of venous abnormalities in diabetic retinopathy. Jpn J Ophthalmol. 1993; 37: 136–142
5. Bek T. A clinicopathological study of venous loops and reduplications in diabetic retinopathy. Acta Ophthalmol Scand. 2002;80(1):69-75
Volume 6 | Issue 8 | 2020 | Endocrinology and Diabetology 52
FIGURE 2: Close-up FFA image LE shows severe nonperfusion areas adjacent to venous loops with enlargement of foveal avascular zone
FIGURE 3: Late staining and leakage from nasal venous loop
FIGURE 4: Worsening of vitreous haemorrhage covering inferior half of the macula still looks emanating from the venous loop
FIGURE 5: Settling subhyaloid haemorrhage inferiorly














































available in print and digital
For all that matters in medicine
For the treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years1


treatment of diabetes mellitus in adults, and children from the age of 6 years1







Prescribing Information:

















®



Toujeo (insulin glargine 300 units/ml)




















before prescribing.
Please refer to Summary of Product Characteristics (SmPC) before prescribing.
Each ml contains 300 units of insulin glargine. SoloStar pen contains 3ml (900 units) of solution for injection. and children from the age of 6 years. Dosage and injection into the abdominal wall, the deltoid or the thigh, once Injection sites must be rotated within a given injection lipodystrophy and cutaneous amyloidosis. The dose regimen response. Do not administer intravenously. In type 1 diabetes to cover mealtime insulin requirements. In patients with units/kg followed by individual dose adjustments. Toujeo medicinal products. Switch between insulin glargine 100 units/ bioequivalent and are not directly interchangeable. When done on a unit to unit basis, but a higher Toujeo dose for plasma glucose levels. When switching from Toujeo (approximately by 20%). Switching from other basal insulins to concomitant anti hyperglycaemic treatment may be required. lifestyle changes, the timing of insulin dose is changed or hyperglycaemia. Toujeo must not be mixed or diluted with monitoring is recommended during a switch and in the initial 1 unit and DoubleStar 2-160 units in steps of 2 units. patient’s previous dose was an odd number then the prefilled pen is recommended for patients requiring at may be diminished in the elderly or patients with renal or Toujeo, dose reduction of basal and bolus insulin needs to be hypoglycaemia. The safety and efficacy of Toujeo in children
Presentation: Toujeo SoloStar and DoubleStar pre-filled pens. Each ml contains 300 units of insulin glargine. SoloStar pen contains 1.5ml (450 units) of solution for injection. DoubleStar pen contains 3ml (900 units) of solution for injection.
Indication: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. Dosage and



unaffected area has been reported to result in hypoglycaemia. Blood glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar pre-filled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There is no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Legal Category: POM. Marketing Authorisation Number: SoloStar 3 Pen pack: EU/1/00/133/034, DoubleStar EU/1/00/133/038. Marketing Authorisation
Contraindications: Hypersensitivity to insulin glargine or improve the traceability of biological medicinal products, be clearly recorded. Toujeo is not the insulin of choice for perform continuous rotation of the injection site to reduce There is a potential risk of delayed insulin absorption and these reactions. A sudden change in the injection site to an
Administration: Toujeo is administered subcutaneously, by injection into the abdominal wall, the deltoid or the thigh, once daily, at any time of the day, preferably at the same time every day. Injection sites must be rotated within a given injection area from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis. The dose regimen (dose and timing) should be adjusted according to individual response. Do not administer intravenously. In type 1 diabetes mellitus, Toujeo must be combined with short-/rapid-acting insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, recommended daily starting dose is 0.2 units/kg followed by individual dose adjustments. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch between insulin glargine 100 units/ ml and Toujeo: Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not directly interchangeable. When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit to unit basis, but a higher Toujeo dose (approximately 10-18%) may be needed to achieve target ranges for plasma glucose levels. When switching from Toujeo to insulin glargine 100 units/ml, the dose should be reduced (approximately by 20%). Switching from other basal insulins to Toujeo: A change of dose and/or timing of the basal insulin and concomitant anti hyperglycaemic treatment may be required. Dose adjustments may also be required if the patient’s weight or lifestyle changes, the timing of insulin dose is changed or other circumstances arise that increase susceptibility to hypo- or hyperglycaemia. Toujeo must not be mixed or diluted with any other insulin or other medicinal products. Close metabolic monitoring is recommended during a switch and in the initial weeks thereafter. SoloStar 1-80 units per single injection in steps of 1 unit and DoubleStar 2-160 units in steps of 2 units. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number then the dose must be increased or decreased by 1 unit. Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Special Populations: Insulin requirements may be diminished in the elderly or patients with renal or hepatic impairment. Paediatric: When switching basal insulin to Toujeo, dose reduction of basal and bolus insulin needs to be considered on an individual basis, in order to minimise the risk of hypoglycaemia. The safety and efficacy of Toujeo in children and adolescents below 6 years of age have not been established. Contraindications: Hypersensitivity to insulin glargine or any excipients. Precautions and Warnings: Traceability: In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Toujeo is not the insulin of choice for treatment of diabetic ketoacidosis. Patients must be instructed to perform continuous rotation of the injection site to reduce the risk of developing lipodystrophy and cutaneous amyloidosis. There is a potential risk of delayed insulin absorption and worsened glycaemic control following insulin injections at sites with these reactions. A sudden change in the injection site to an

References:1. Toujeo® Summary of Product Characteristics






































































Date of preparation: September 2020 | MAT-IE-2001062 (v1.0)
Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2020.



















unaffected area has been reported to result in hypoglycaemia. Blood glucose monitoring is recommended after the change in the injection site, and dose adjustment of antidiabetic medications may be considered. Hypoglycaemia: In case of insufficient glucose control or a tendency to hyper/hypoglycaemic episodes, the patient’s adherence to the prescribed treatment regimen, injection sites and proper injection technique and all other relevant factors must be reviewed before dose adjustment is considered. Particular caution should be exercised, and intensified blood glucose monitoring is advisable for patients in whom hypoglycaemic episodes might be of clinical relevance and in those where dose adjustments may be required. Warning signs of hypoglycaemia may be changed, less pronounced or absent in certain risk groups, potentially resulting in severe hypoglycaemia and loss of consciousness. Risk groups include patients in whom glycaemic control is markedly improved, hypoglycaemia develops gradually, an autonomic neuropathy is present, or who are elderly. The prolonged effect of subcutaneous insulin glargine may delay recovery from hypoglycaemia. Intercurrent illness: Requires intensified metabolic monitoring and often it is necessary to adjust the insulin dose. Insulin antibodies: administration may cause insulin antibodies to form. Use with pioglitazone: Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. Medication errors: Insulin labels must always be checked before each injection to avoid errors between Toujeo and other insulins. Patients must be instructed to never use a syringe to remove Toujeo from the SoloStar or DoubleStar pre-filled pen, A new sterile needle must be attached before each injection. Needles must not be re-used. Pregnancy and lactation: There is no data from exposed pregnancies in controlled clinical trials. However, there is a large amount of data on use of insulin glargine 100 units/ml in pregnant women indicating no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity. The use of Toujeo may be considered during pregnancy, if clinically needed. Careful monitoring of glucose control is essential. It is unknown if insulin glargine is excreted in breast milk. Interactions: Substances that affect glucose metabolism may require adjustment of insulin glargine. Adverse Reactions: Very common: Hypoglycaemia. Prolonged or severe hypoglycaemia may be life-threatening. Common: Lipohypertrophy, injection site reactions, including redness, pain, itching, hives, swelling, or inflammation. Legal Category: POM. Marketing Authorisation Number: SoloStar 3 Pen pack: EU/1/00/133/034, DoubleStar EU/1/00/133/038. Marketing Authorisation Holder: Sanofi Aventis Deutschland GmbH, D-65926 Frankfurt am Main, Germany. Further information is available from: Medical Information, Sanofi 18 Riverwalk, Citywest Business Campus, Dublin 24 or contact IEmedinfo@sanofi.com. Date of preparation: July 2020.
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
Adverse events should be reported. Reporting forms and information can be found at www.hpra.ie; email: medsafety@hpra.ie Adverse events should also be reported to Sanofi Ireland Ltd. Tel: 01 403 5600. Alternatively, send via email to IEPharmacovigilance@sanofi.com
(v1.0)
















































































































































































































































































