Removal of 17β-Estradiol by Activated Charcoal Supported Titanate Nanotubes (TNTs@AC) through Initial Adsorption and Subsequent Photo-Degradation: Intermediates, DFT calculation, and Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of TNTs@AC
2.3. Experimental Methods: Adsorption Kinetic and Isotherm, Photoregeneration
2.4. Characterizations
2.5. Analytical Methods
2.6. DFT Calculation Method
3. Results and Discussion
3.1. Material Characterizations
3.2. Adsorption Kinetics of E2 by TNTs@AC
3.3. Adsorption Isotherms of E2 by Various Materials
3.4. Photoregeneration of TNTs@AC
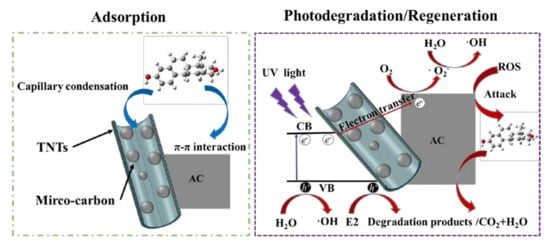
3.5. Removal Mechanism of E2 by TNTs@AC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ejhed, H.; Fång, J.; Hansen, K.; Graae, L.; Rahmberg, M.; Magnér, J.; Dorgeloh, E.; Płaza, G.A. The effect of hydraulic retention time in onsite wastewater treatment and removal of pharmaceuticals, hormones and phenolic utility substances. Sci. Total Environ. 2018, 618, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.Z.; Shamsuddin, A.S.; Praveena, S.M. Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environ. Int. 2014, 69, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Liu, S.; Hu, X.; Zeng, G.; Hu, X.; Liu, S.; Liu, S.; Huang, B.; Li, M. Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem. Eng. J. 2017, 308, 597–605. [Google Scholar] [CrossRef]
- Shyu, C.; Cavileer, T.D.; Nagler, J.J.; Ytreberg, F.M. Computational estimation of rainbow trout estrogen receptor binding affinities for environmental estrogens. Toxicol. Appl. Pharmacol. 2011, 250, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; Chang, J.; Zhao, H.; Liu, W.; Dang, C.; Tong, M.; Ni, J.; Zhang, B. Sea-Buckthorn-Like MnO2 Decorated Titanate Nanotubes with Oxidation Property and Photocatalytic Activity for Enhanced Degradation of 17β-Estradiol under Solar Light. ACS Appl. Energy Mater. 2018, 1, 2123–2133. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Liu, S.; Jiang, L.; Tan, X.; Zeng, G.; Li, M.; Liu, S.; Tian, S.; Fang, Y. Activated magnetic biochar by one-step synthesis: Enhanced adsorption and coadsorption for 17β-estradiol and copper. Sci. Total Environ. 2018, 639, 1530–1542. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Shon, H.; Vigneswaran, S.; Snyder, S.A. Effluent Organic Matter (EfOM) in Wastewater: Constituents, Effects, and Treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Leech, D.M.; Snyder, M.T.; Wetzel, R.G. Natural organic matter and sunlight accelerate the degradation of 17ß-estradiol in water. Sci. Total Environ. 2009, 407, 2087–2092. [Google Scholar] [CrossRef]
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.L. Removal of estrone and 17β-estradiol from water by adsorption. Water Res. 2005, 39, 3991–4003. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Xie, W.; Liu, W.; Liu, X.; Zhao, D. Sorption of dispersed petroleum hydrocarbons by activated charcoals: Effects of oil dispersants. Environ. Pollut. 2019, 256, 113416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Zeng, G.; Liu, S.; Hu, X.; Zhou, L.; Tan, X.; Liu, N.; Li, M.; Wen, J. Adsorption of estrogen contaminants (17β-estradiol and 17α-ethynylestradiol) by graphene nanosheets from water: Effects of graphene characteristics and solution chemistry. Chem. Eng. J. 2018, 339, 296–302. [Google Scholar] [CrossRef]
- Jung, C.; Son, A.; Her, N.; Zoh, K.-D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Dang, C.; Sun, F.; Jiang, H.; Huang, T.; Liu, W.; Chen, X.; Ji, H. Pre-accumulation and in-situ destruction of diclofenac by a photo-regenerable activated carbon fiber supported titanate nanotubes composite material: Intermediates, DFT calculation, and ecotoxicity. J. Hazard. Mater. 2020, 400, 123225. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Borthwick, A.G.; Wang, Y.; Yin, X.; Li, X.; Ni, J. Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: Competition and effect of inorganic ions. Sci. Total Environ. 2013, 456, 171–180. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Zhang, W.; Wei, J.; Chen, B.; Wang, C. Sediments inhibit adsorption of 17β-estradiol and 17α-ethinylestradiol to carbon nanotubes and graphene oxide. Environ. Sci. Nano 2017, 4, 1900–1910. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Mei, J.; Sarina, S.; Wu, Z.; Liao, T.; Yan, C.; Sun, Z. Strongly interfacial-coupled 2D-2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 2020, 394, 122529. [Google Scholar] [CrossRef]
- Trellu, C.; Mousset, E.; Pechaud, Y.; Huguenot, D.; Van Hullebusch, E.D.; Esposito, G.; Oturan, N. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. J. Hazard. Mater. 2016, 306, 149–174. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2019, 701, 135023. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, X.; Borthwick, A.G.L.; Wang, Y.; Ni, J. Dual-Enhanced Photocatalytic Activity of Fe-Deposited Titanate Nanotubes Used for Simultaneous Removal of As(III) and As(V). ACS Appl. Mater. Interfaces 2015, 7, 19726–19735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Wang, T.; Ma, R.; Liu, W.; Cui, F.; Sun, W. Influences of isolated fractions of natural organic matter on adsorption of Cu(II) by titanate nanotubes. Sci. Total Environ. 2019, 650, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Cai, Z.; Fu, J.; Sun, X.; Sun, W.; Chen, L.; Zhang, D.; Liu, W. Synergistic adsorption of Cu(II) and photocatalytic degradation of phenanthrene by a jaboticaba-like TiO2/titanate nanotube composite: An experimental and theoretical study. Chem. Eng. J. 2019, 358, 1155–1165. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Liu, W.; Li, Z.; He, K. Efficient removal of dyes from dyeing wastewater by powder activated charcoal/titanate nanotube nanocomposites: Adsorption and photoregeneration. Environ. Sci. Pollut. Res. 2019, 26, 10263–10273. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cai, Z.; Zhao, X.; Wang, T.; Li, F.; Zhao, D. High-Capacity and Photoregenerable Composite Material for Efficient Adsorption and Degradation of Phenanthrene in Water. Environ. Sci. Technol. 2016, 50, 11174–11183. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01. Available online: https://gaussian.com/citation/ (accessed on 22 July 2020).
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D graphitic carbon nitride/titanate nanotubes heterostructure for efficient photocatalysis of sulfamethazine under solar light: Catalytic “hot spots” at the rutile–anatase–titanate interfaces. Appl. Catal. B Environ. 2020, 263, 118357. [Google Scholar] [CrossRef]
- Ma, M.; Chen, L.; Zhao, J.; Liu, W.; Ji, H. Efficient activation of peroxymonosulfate by hollow cobalt hydroxide for degradation of ibuprofen and theoretical study. Chin. Chem. Lett. 2019, 30, 2191–2195. [Google Scholar] [CrossRef]
- Liu, X.; Ji, H.; Li, S.; Liu, W. Graphene modified anatase/titanate nanosheets with enhanced photocatalytic activity for efficient degradation of sulfamethazine under simulated solar light. Chemosphere 2019, 233, 198–206. [Google Scholar] [CrossRef]
- Manzetti, S.; Lu, T. The geometry and electronic structure of Aristolochic acid: Possible implications for a frozen resonance. J. Phys. Org. Chem. 2013, 26, 473–483. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Ma, J.; Li, F.; Qian, T.; Liu, H.; Liu, W.; Zhao, D. Natural organic matter resistant powder activated charcoal supported titanate nanotubes for adsorption of Pb(II). Chem. Eng. J. 2017, 315. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ji, H.; Zhu, Y.; Duan, J.; Liu, W.; Zhao, D. Reductive immobilization and long-term remobilization of radioactive pertechnetate using bio-macromolecules stabilized zero valent iron nanoparticles. Chin. Chem. Lett. 2019, 30, 2163–2168. [Google Scholar] [CrossRef]
- Ji, H.; Zhu, Y.; Liu, W.; Bozack, M.J.; Qian, T.; Zhao, D. Sequestration of pertechnetate using carboxymethyl cellulose stabilized FeS nanoparticles: Effectiveness and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 373–380. [Google Scholar] [CrossRef]
- Ji, H.; Gong, Y.; Duan, J.; Zhao, D.; Liu, W. Degradation of petroleum hydrocarbons in seawater by simulated surface-level atmospheric ozone: Reaction kinetics and effect of oil dispersant. Mar. Pollut. Bull. 2018, 135, 427–440. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Zhao, D.; Pignatello, J.J.; White, J.C.; Braida, W.; Ferrandino, F. Dual-mode modeling of competitive and concentration-dependent sorption and desorption kinetics of polycyclic aromatic hydrocarbons in soils. Water Resour. Res. 2001, 37, 2205–2212. [Google Scholar] [CrossRef]
- Liao, Z.; Nguyen, M.N.; Wan, G.; Xie, J.; Ni, L.; Qi, J.; Li, J.; Schafer, A.I. Low pressure operated ultrafiltration membrane with integration of hollow mesoporous carbon nanospheres for effective removal of micropollutants. J. Hazard. Mater. 2020, 397, 122779. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, X.; Wang, T.; Zhao, D.; Ni, J. Adsorption of U(VI) by multilayer titanate nanotubes: Effects of inorganic cations, carbonate and natural organic matter. Chem. Eng. J. 2016, 286, 427–435. [Google Scholar] [CrossRef]







| Models | Parameters | Materials | ||
|---|---|---|---|---|
| TNTs@AC | AC | TNTs | ||
| Liner model | Kd (L/g) | 26.46 | 8.64 | 0.426 |
| R2 | 0.542 | 0.762 | 0.697 | |
| Langmuir model | Qmax (mg/g) | 2.78 | 2.57 | 0.16 |
| b (L/mg) | 102.89 | 17.20 | 9.60 | |
| R2 | 0.977 | 0.949 | 0.991 | |
| Freundlich model | KF (mg/g·(L/mg)1/n) | 6.82 | 3.94 | 0.23 |
| n | 2.53 | 2.18 | 1.95 | |
| R2 | 0.913 | 0.989 | 0.970 | |
| Dual-mode model | Kd (L/g) | 10.84 | 4.83 | 0.006 |
| QL (mg/g) | 1.44 | 0.89 | 0.16 | |
| b (L/mg) | 511.95 | 126.18 | 9.49 | |
| R2 | 0.995 | 0.996 | 0.995 | |
| Models | Parameters | Materials |
|---|---|---|
| TNTs@AC | ||
| Liner model | Kd (L/g) | 10.729 |
| R2 | 0.669 | |
| Langmuir model | Qmax (mg/g) | 4.82 |
| b (L/mg) | 23.58 | |
| R2 | 0.958 | |
| Freundlich model | KF (mg/g·(L/mg)1/n) | 2.87 |
| n | 5.79 | |
| R2 | 0.991 | |
| Dual-mode model | Kd (L/g) | 5.91 |
| QL (mg/g) | 1.87 | |
| b (L/mg) | 239.36 | |
| R2 | 0.995 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Pan, B.; Ji, H.; Liu, W. Removal of 17β-Estradiol by Activated Charcoal Supported Titanate Nanotubes (TNTs@AC) through Initial Adsorption and Subsequent Photo-Degradation: Intermediates, DFT calculation, and Mechanisms. Water 2020, 12, 2121. https://doi.org/10.3390/w12082121
Huang T, Pan B, Ji H, Liu W. Removal of 17β-Estradiol by Activated Charcoal Supported Titanate Nanotubes (TNTs@AC) through Initial Adsorption and Subsequent Photo-Degradation: Intermediates, DFT calculation, and Mechanisms. Water. 2020; 12(8):2121. https://doi.org/10.3390/w12082121
Chicago/Turabian StyleHuang, Taobo, Baozhu Pan, Haodong Ji, and Wen Liu. 2020. "Removal of 17β-Estradiol by Activated Charcoal Supported Titanate Nanotubes (TNTs@AC) through Initial Adsorption and Subsequent Photo-Degradation: Intermediates, DFT calculation, and Mechanisms" Water 12, no. 8: 2121. https://doi.org/10.3390/w12082121